High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites
Abstract
:1. Introduction
2. Results
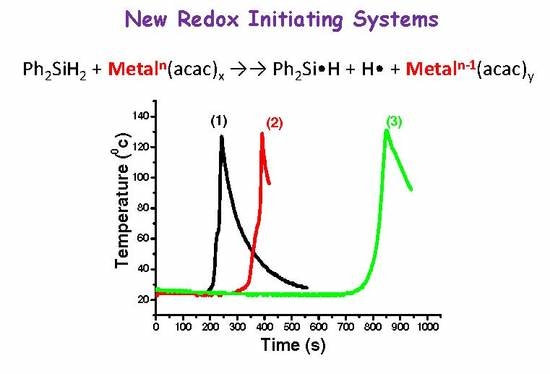
2.1. Redox Initiating Systems (RISs) using DPS/Metal Complex Combination
2.2. Potential Photoactivation of the New Proposed RIS (DPS/Mn(acac)2)
2.3. Stability upon Storage
2.4. Application to the Preparation of Composites
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Two Cartridges System Used for Redox Experiments
4.3. Redox Polymerization in Bulk Followed by Optical Pyrometry
4.4. RT-FTIR Spectroscopy
4.5. Electron Spin Resonance (ESR) Spin Trapping (ESR-ST)
4.6. Cyclic Voltammetry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yagci, Y.; Mishra, M. Handbook of Vinyl Polymers - Radical Polymerization and Technology; Wiley: New York, NY, USA, 2009; pp. 307–344. [Google Scholar]
- Green, W.A. Industrial Photoinitiators: A Technical Guide; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.A.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Kwon, T.-Y.; Bagheri, R.; Kim, Y.K.; Kim, K.-H.; Burrow, M.F. Cure mechanisms in materials for use in esthetic dentistry. J. Investig. Clin. Dent. 2012, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Achilias, D.S.; Kostidou, N.C. Copolymerization kinetics of dental dimethacrylate resins initiated by a benzoyl peroxide/amine redox system. J. Appl. Polym. Sci. 2008, 109, 515–524. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S.; Karava, O. Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopedic Dimethacrylate Monomers: Effect of the Amine and Monomer Chemical Structure. Macromolecules 2006, 39, 2072–2080. [Google Scholar] [CrossRef]
- Han, D.; Meng, Z.; Wu, D.; Zhang, C.; Zhu, H. Thermal properties of carbon black aqueous nanofluids for solar absorption. Nanoscale Res. Lett. 2011, 6, 457. [Google Scholar] [CrossRef] [Green Version]
- Achilias, D.S.; Sideridou, I. Study of the Effect of Two BPO/Amine Initiation Systems on the Free-Radical Polymerization of MMA Used in Dental Resins and Bone Cements. J. Macromol. Sci. Part A 2002, 39, 1435–1450. [Google Scholar] [CrossRef]
- Wilson, G.O.; Henderson, J.W.; Caruso, M.M.; Blaiszik, B.; McIntire, P.J.; Sottos, N.R.; White, S.R.; Moore, J.S. Evaluation of peroxide initiators for radical polymerization-based self-healing applications. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 2698–2708. [Google Scholar] [CrossRef]
- Snider, B.B. Manganese(III)-Based Oxidative Free-Radical Cyclizations. Chem. Rev. 1996, 96, 339–364. [Google Scholar] [CrossRef]
- Kastning, E.-G.; Naarmann, H.; Reis, H.; Berding, C. Metal Chelates as Polymerization Initiators. Angew. Chem. Int. Ed. 1965, 4, 322–327. [Google Scholar] [CrossRef]
- Endo, K.; Yachi, A. Molecular-weight-controlled polymerization of styrene with Mn(acac)3 in combination with organic halides. Polym. Bull. 2001, 46, 363–369. [Google Scholar] [CrossRef]
- Endo, K.; Yachi, A. Polymerization of Methyl Methacrylate with Mn(acac)3 in the Presence of Organic Halides. Possibility of Molecular Weight Control of Polymer. Polym. J. 2002, 34, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Nayak, P.L.; Samal, R.K.; Nayak, M.C. Aqueous Polymerization of Acrylonitrile Initiated by the Mn3+/Citric Acid Redox System. Eur. Polym. J. 1978, 14, 287–290. [Google Scholar] [CrossRef]
- Caille, J.-R.; Debuigne, A.; Jérôme, C. Quinone transfer radical polymerization (QTRP) of styrene: Catalysis by different metal complexes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2723–2733. [Google Scholar] [CrossRef]
- Van Gorkum, R.; Bouwman, E.; Reedijk, J. Fast Autoxidation of Ethyl Linoleate Catalyzed by [Mn(acac)3] and Bipyridine: A Possible Drying Catalyst for Alkyd Paints. Inorg. Chem. 2004, 43, 2456–2458. [Google Scholar] [CrossRef]
- Bouwman, E.; Van Gorkum, R. A study of new manganese complexes as potential driers for alkyd paints. J. Coat. Technol. Res. 2007, 4, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev. 2013, 114, 557–589. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Al Mousawi, A.; Graff, B.; Gigmes, D.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Mechanosynthesized copper(i) complex based initiating systems for redox polymerization: Towards upgraded oxidizing and reducing agents. Polym. Chem. 2017, 8, 5884–5896. [Google Scholar] [CrossRef]
- Garra, P.; Brunel, D.; Noirbent, G.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Sidorkin, V.F.; Dumur, F.; Duché, D.; Gigmes, D.; et al. Ferrocene-based (photo)redox polymerization under long wavelengths. Polym. Chem. 2019, 10, 1431–1441. [Google Scholar] [CrossRef]
- Garra, P.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Monnier, V.; Dietlin, C.; Gigmes, D.; Lalevée, J.; Fouassier, J. Metal Acetylacetonate–Bidentate Ligand Interaction (MABLI) as highly efficient free radical generating systems for polymer synthesis. Polym. Chem. 2018, 9, 1371–1378. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. A New Highly Efficient Amine-Free and Peroxide-Free Redox System for Free Radical Polymerization under Air with Possible Light Activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Garra, P.; Baralle, A.; Graff, B.; Schrodj, G.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.-P.; Lalevée, J. Radical Cations in Versatile High-Performance Initiating Systems for Thermal, Redox, and Photopolymerizations. Macromolecules 2018, 51, 8899–8911. [Google Scholar] [CrossRef]
- Wang, D.; Garra, P.; Szillat, F.; Fouassier, J.P.; Lalevée, J. Silane Based Redox Initiating Systems: Toward a Safer Amine-Free, Peroxide-Free, and Metal-Free Approach. Macromolecules 2019, 52, 3351–3358. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Chany, A.C.; El-Roz, M.; Souane, R.; Graff, B.; Allonas, X.; Fouassier, J.P. Silyl Radical Chemistry and Conventional Photoinitiators: A Route for the Design of Efficient Systems. Macromolecules 2009, 42, 6031–6037. [Google Scholar]
- Janzen, E.G.; Blackburn, B.J. Detection and identification of short-lived free radicals by electron spin resonance trapping techniques (spin trapping). Photolysis of organolead, -tin, and -mercury compounds. J. Am. Chem. Soc. 1969, 91, 4481–4490. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| System | Gel Time (s) | Maximum T °C | Surface Curing | Final C=C Conversion (%) | Ered (V) | ΔG[eV] |
|---|---|---|---|---|---|---|
| Mn(acac)2 / DPS 1/1 wt% | 110 | 140 | Tack-free | 98% | −1.07 | 2.47 |
| Cu(AAEMA)2 / DPS 1/1 wt% | 380 | 130 | Tack-free | 90% | −0.65 | 2.05 |
| Fe(acac)3 / DPS 1/1 wt% | 900 | 45 | Tacky | n.d. | n.d. | n.d. |
| Mn(acac)3 / DPS 1/1 wt% | 155 | 142 | Tack-free | 98% | −0.85 | 2.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arar, A.; Mokbel, H.; Dumur, F.; Lalevée, J. High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites. Molecules 2020, 25, 1602. https://doi.org/10.3390/molecules25071602
Arar A, Mokbel H, Dumur F, Lalevée J. High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites. Molecules. 2020; 25(7):1602. https://doi.org/10.3390/molecules25071602
Chicago/Turabian StyleArar, Ahmad, Haifaa Mokbel, Frédéric Dumur, and Jacques Lalevée. 2020. "High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites" Molecules 25, no. 7: 1602. https://doi.org/10.3390/molecules25071602
APA StyleArar, A., Mokbel, H., Dumur, F., & Lalevée, J. (2020). High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites. Molecules, 25(7), 1602. https://doi.org/10.3390/molecules25071602