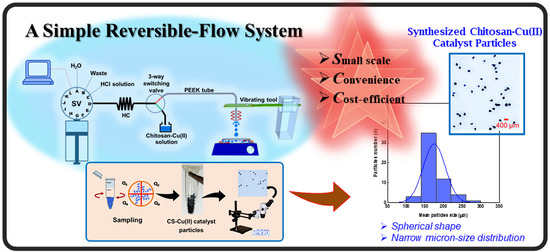
Development of a Simple Reversible-Flow Method for Preparation of Micron-Size Chitosan-Cu(II) Catalyst Particles and Their Testing of Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Sampling Procedure for Measurement of Particle Size of CS-Cu(II) Catalyst Particles
2.2. Investigation of Flow Parameters
2.2.1. Effect of Chitosan Content
2.2.2. Effect of NaOH Concentration
2.2.3. Effect of Flow Rate of the CS-Cu(II) Reactant Solution
2.3. Comparison of Methods for Synthesizing CS-Cu(II) Catalyst Particles: Flow Methods vs. Manual Addition
2.4. Characterization of CS-Cu(II) Catalyst Particles
2.5. Assessment of the Catalytic Activity of the Chitosan-Cu(II) Particles in the Reduction Reaction of p-Nitrophenol with Excess Borohydride
3. Materials and Methods
3.1. Chemicals and Preparation of Chitosan and Cu(II) Solutions
3.2. Preparation of CS-Cu(II) Catalyst Particles
3.2.1. Configuration and Operation of the Flow System
3.2.2. Manual Dropping Method
3.3. Sampling and Measurement of Particle Size of Synthesized CS-Cu(II) Catalyst
3.4. Method of Characterization of CS-Cu(II) Catalyst Particles
3.5. Procedure for Measuring Activity of CS-Cu(II) Particles Using Reduction Reaction of p-Nitrophenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parshetti, G.K.; Suryadharma, M.S.; Pham, T.P.T.; Mahmood, R.; Balasubramanian, R. Heterogeneous catalyst-assisted thermochemical conversion of food waste biomass into 5-hydroxymethylfurfural. Bioresour. Technol. 2015, 178, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Thomas, J.M.; Greenhill-Hooper, M.; Ley, S.V.; Almeida Paz, F.A. Facile, One-step production of niacin (Vitamin b3) and other nitrogen-containing pharmaceutical chemicals with a single-site heterogeneous catalyst. Chem. Eur. 2008, 14, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.Y.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on chitosan: A recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem. 2014, 16, 2122–2127. [Google Scholar] [CrossRef]
- Li, M.; Su, Y.J.; Hu, J.; Geng, H.J.; Wei, H.; Yang, Z.; Zhang, Y.F. Hydrothermal synthesis of porous copper microspheres towards efficient 4-nitrophenol reduction. Mater. Res. Bull. 2016, 83, 329–335. [Google Scholar] [CrossRef]
- Fisch, A.G.; dos Santos, J.H.Z.; Secchi, A.R.; Cardozo, N.S.M. Heterogeneous catalysts for olefin polymerization: Mathematical model for catalyst particle fragmentation. Ind. Eng. Chem. Res. 2015, 54, 11997–12010. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Downing, R.S. Heterogeneous catalytic transformations for environmentally friendly production. Appl. Catal. A 1999, 189, 163–183. [Google Scholar] [CrossRef]
- Ding, Y.B.; Zhu, L.H.; Huang, A.Z.; Zhao, X.R.; Zhang, X.Y.; Tang, H.Q. A heterogeneous Co3O4-Bi2O3 composite catalyst for oxidative degradation of organic pollutants in the presence of peroxymonosulfate. Catal. Sci. Technol. 2012, 2, 1977–1984. [Google Scholar] [CrossRef]
- Fedorczyk, A.; Ratajczak, J.; Kuzmych, O.; Skompska, M. Kinetic studies of catalytic reduction of 4-nitrophenol with NaBH4 by means of Au nanoparticles dispersed in a conducting polymer matrix. J. Solid State Electrochem. 2015, 19, 2849–2858. [Google Scholar] [CrossRef]
- Prusse, U.; Dalluhn, J.; Breford, J.; Vorlop, K.D. Production of spherical beads by JetCutting. Chem. Eng. Technol. 2000, 23, 1105–1110. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agent on the properties of spray dried microspheres. J. Microencapsul. 2005, 22, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, I.; Niemeyer, P.; Yusufoglu, Y.; Gurikov, P.; Milow, B.; Smirnova, I. Polysaccharide-Based Aerogel Bead Production via Jet Cutting Method. Materials 2018, 11, 1287. [Google Scholar] [CrossRef] [Green Version]
- Barreiro-Iglesias, R.; Coronilla, R.; Concheiro, A.; Alvarez-Lorenzo, C. Preparation of chitosan beads by simultaneous cross-linking/insolubilisation in basic pH: Rheological optimisation and drug loading/release behaviour. Eur. J. Pharm. Sci. 2005, 24, 77–84. [Google Scholar] [CrossRef]
- Kramareva, N.V.; Stakheev, A.Y.; Tkachenko, O.P.; Klementiev, K.V.; Grunert, W.; Finashina, E.D.; Kustov, L.M. Heterogenized palladium chitosan complexes as potential catalysts in oxidation reactions: Study of the structure. J. Mol. Catal. A. Chem. 2004, 209, 97–106. [Google Scholar] [CrossRef]
- Makhmalzadeh, B.; Moshtaghi, F.; Rahim, F.; Aakhgari, A. Preparation and evaluation of sodium diclofenac loadedchitosan controlled release microparticles using factorial design. Int. J. Drug Dev. Res. 2010, 2, 468–475. [Google Scholar]
- Xu, S.; Wang, Z.; Gao, Y.; Zhang, S.; Wu, K. Adsorption of Rare Earths(Ⅲ) Using an efficient sodium alginate hydrogel cross-linked with poly-γ-glutamate. PLoS ONE 2015, 10, e0124826. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Hu, D.; Yan, Q.; Wang, Z.; Li, S.; Chen, C.; Xue, Q. Swelling induced regeneration of TiO2-impregnated chitosan adsorbents under visible light. Carbohydr. Polym. 2016, 140, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, R.F.N.; Fajardo, A.R. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arab. J. Chem. 2020, 13, 2183–2194. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Trojanowicz, M. Flow chemistry in contemporary chemical sciences: A real variety of its applications. Molecules 2020, 25, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.-P.; Lin, L.; Li, Q.; Zhou, Z.; Wang, T.; Russell, C.K.; Adidharma, H.; Xu, Z.; Yao, Y.-G.; Fan, M. Recent progress in improving the stability of copper-based catalysts for hydrogenation of carbon–oxygen bonds. Catal. Sci. Technol. 2018, 8, 3428–3449. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Chutimasakul, T.; Na Nakhonpanom, P.; Tirdtrakool, W.; Intanin, A.; Bunchuay, T.; Chantiwas, R.; Tantirungrotechai, J. Uniform Cu/chitosan beads as green and reusable catalyst for facile synthesis of imines via oxidative coupling reaction. Unpublished work. 2020. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Bagherzadeh, M.; Safari, R. Immobilization of copper nanoparticles on perlite: Green synthesis, characterization and catalytic activity on aqueous reduction of 4-nitrophenol. J. Mol. Catal. A: Chem. 2015, 400, 22–30. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, D.-H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664–669. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J. Alloy. Compd. 2016, 680, 309–314.4. [Google Scholar] [CrossRef]
- Kumar, A.; Belwal, M.; Maurya, R.R.; Mohan, V.; Vishwanathan, V. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by Au/AC nanocatalysts. Mat. Sci. Eng.Technol. 2019, 2, 526–531. [Google Scholar] [CrossRef]
- Hasan, K.; Shehadi, I.A.; Al-Bab, N.D.; Elgamouz, A. Magnetic chitosan-supported silver nanoparticles: A heterogeneous catalyst for the reduction of 4-nitrophenol. Catalysts 2019, 9, 839. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Step | Port | Flow Rate (µL s−1) | Operation (Volume/µL) | Description |
|---|---|---|---|---|
| Aspirate H2O carrier into syringe pump (repeat steps 1–2 twice) | ||||
| 1 | 1 | 50 | Aspirate (5000 µL) | Syringe pump valve in; aspirate H2O. |
| 2 | 4 | 50 | Dispense (5000 µL) | Syringe pump valve out; dispense H2O. |
| Aspirate air, CS-Cu(II) catalyst solution into holding coil | ||||
| 3 | 4 | 10 | Aspirate (200 µL) | Switch 3-port switching valve; dispense air plug. |
| 4 | 4 | 10 | Aspirate (4000 µL) | Aspirate plug of CS-Cu(II) catalyst solution. |
| Dispense CS-Cu(II) catalyst solution, air to the tip of tubing | ||||
| 5 | 4 | 10 | Dispense (4200 µL) | Switch 3-port switching valve; dispense CS-Cu(II) catalyst solution and air plugs. |
| Cleaning of holding coil by 0.2 M HCl solution and H2O (repeat steps 6–9 twice) | ||||
| 6 | 3 | 50 | Aspirate (5000 µL) | Syringe pump valve in; aspirate 0.2 M HCl solution plug. |
| 7 | 4 | 50 | Empty | Syringe pump valve out; dispense 0.2 M HCl solution plug. |
| 8 | 1 | 50 | Aspirate (5000 µL) | Syringe pump valve in; aspirate H2O plug. |
| 9 | 1 | 50 | Empty | Syringe pump valve out; dispense H2O plug. |
| Parameter | Studied Range | Selected Value |
|---|---|---|
| 1. Concentration of chitosan (% w/v) | 0.5–1.5 | 1.5 |
| 2. Concentration of copper acetate (M) | 1–3 | 2 |
| 3. Concentration of NaOH (M) | 1–3 | 2 |
| 4. Flow rate (µL s−1) | 10–50 | 10 |
| Parameter | Manual Method | Flow Method Without Vibration | Flow Method with Vibrating Rod |
|---|---|---|---|
| 1. Operation mode | Manual | Computer control | Computer control |
| 2. Mean particles size (n = 50) | 880 ± 70 µm | 780 ± 20 µm | 180 ± 30 µm |
| 3. Size distribution | 810–950 µm | 760–800 µm | 150–210 µm |
| 4. Shape | Quasi-spherical | Spherical | Spherical |
| 5. Throughput | 40 drops min−1 | 60 drops min−1 | 100 drops min−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intanin, A.; Inpota, P.; Chutimasakul, T.; Tantirungrotechai, J.; Wilairat, P.; Chantiwas, R. Development of a Simple Reversible-Flow Method for Preparation of Micron-Size Chitosan-Cu(II) Catalyst Particles and Their Testing of Activity. Molecules 2020, 25, 1798. https://doi.org/10.3390/molecules25081798
Intanin A, Inpota P, Chutimasakul T, Tantirungrotechai J, Wilairat P, Chantiwas R. Development of a Simple Reversible-Flow Method for Preparation of Micron-Size Chitosan-Cu(II) Catalyst Particles and Their Testing of Activity. Molecules. 2020; 25(8):1798. https://doi.org/10.3390/molecules25081798
Chicago/Turabian StyleIntanin, Apichai, Prawpan Inpota, Threeraphat Chutimasakul, Jonggol Tantirungrotechai, Prapin Wilairat, and Rattikan Chantiwas. 2020. "Development of a Simple Reversible-Flow Method for Preparation of Micron-Size Chitosan-Cu(II) Catalyst Particles and Their Testing of Activity" Molecules 25, no. 8: 1798. https://doi.org/10.3390/molecules25081798
APA StyleIntanin, A., Inpota, P., Chutimasakul, T., Tantirungrotechai, J., Wilairat, P., & Chantiwas, R. (2020). Development of a Simple Reversible-Flow Method for Preparation of Micron-Size Chitosan-Cu(II) Catalyst Particles and Their Testing of Activity. Molecules, 25(8), 1798. https://doi.org/10.3390/molecules25081798