Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis
Abstract
:1. Introduction
2. Results
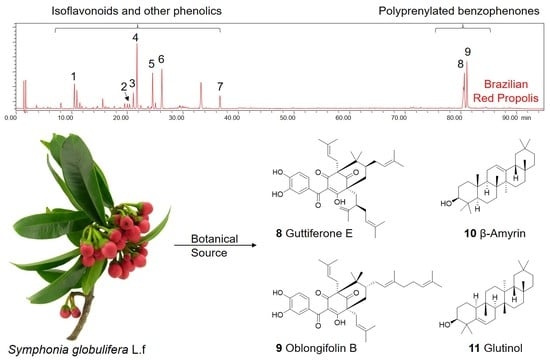
2.1. Isolation of Polyprenylated Benzophenones from Symphonia globulifera and BRP
2.2. The Botanical Sources of Brazilian Red Propolis
3. Discussion
4. Material and Methods
4.1. Field Survey
4.2. Extraction and Isolation of Triterpenoids and Polyprenylated Benzophenones
4.3. HPLC Analyses of Botanical Sources and BRP
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salatino, A.; Salatino, M.L.F. Brazilian red propolis: Legitimate name of the plant resin source. MOJ Food Process. Technol. 2018, 6, 21–22. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evidence-Based Complement. Altern. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.B.; Rosalen, P.L.; Cury, J.A.; Ikegaki, M.; Souza, V.C.; Esteves, A.; Alencar, S.M. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evidence-Based Complement. Altern. Med. 2008, 5, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography–photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.G.; dos Santos Arruda, R.E.; da Cruz Almeida, E.T.; dos Santos Oliveira, J.M.; Basílio-Júnior, I.D.; Celerino de Moraes Porto, I.C.; Rodrigues Sabino, A.; Tonholo, J.; Gray, A.; Ebel, R.A.E.; et al. Comprehensive multivariate correlations between climatic effect, metabolite-profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rufatto, L.C.; dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Roesch Ely, M.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pac. J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Fasolo, D.; Bergold, A.M.; von Poser, G.; Teixeira, H.F. Determination of benzophenones in lipophilic extract of Brazilian red propolis, nanotechnology-based product and porcine skin and mucosa: Analytical and bioanalytical assays. J. Pharm. Biomed. Anal. 2016, 124, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Laguna Miorin, P.; Da, F.; Pasin, R.; Tsvetkova, I. Bioactive constituents of brazilian red propolis. Evidence-Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.S.; de Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Blunt, J.W.; Munro, M.H.G.; Fuller, R.W.; McKee, T.C.; Cardellina, J.H.; McMahon, J.B.; Cragg, G.M.; Boyd, M.R. The guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron 1992, 48, 10093–10102. [Google Scholar] [CrossRef]
- Li, J.; Gao, R.; Zhao, D.; Huang, X.; Chen, Y.; Gan, F.; Liu, H.; Yang, G. Separation and preparation of xanthochymol and guttiferone E by high performance liquid chromatography and high speed counter-current chromatography combined with silver nitrate coordination reaction. J. Chromatogr. A 2017, 1511, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hamed, W.; Brajeul, S.; Mahuteau-Betzer, F.; Thoison, O.; Mons, S.; Delpech, B.; Van Hung, N.; Sévenet, T.; Marazano, C.; Oblongifolins, A.-D. Polyprenylated benzoylphloroglucinol derivatives from Garcinia oblongifolia. J. Nat. 2006, 69, 774–777. [Google Scholar]
- Da Paz Lima, M.; De Campos Braga, P.A.; Macedo, M.L.; Da Silva, M.F.D.G.F.; Ferreira, A.G.; Fernandes, J.B.; Vieira, P.C. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia, and Dacryodes hopkinsii: Chemosystematic implications. J. Braz. Chem. Soc. 2004, 15, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Mahato, S.B.; Das, M.C.; Sahu, N.P. Triterpenoids of Scoparia dulcis. Phytochemistry 1981, 20, 171–173. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). South African J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–11 are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.C.A.S.d.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. https://doi.org/10.3390/molecules25092060
Ccana-Ccapatinta GV, Mejía JAA, Tanimoto MH, Groppo M, Carvalho JCASd, Bastos JK. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules. 2020; 25(9):2060. https://doi.org/10.3390/molecules25092060
Chicago/Turabian StyleCcana-Ccapatinta, Gari Vidal, Jennyfer Andrea Aldana Mejía, Matheus Hikaru Tanimoto, Milton Groppo, Jean Carlos Andrade Sarmento de Carvalho, and Jairo Kenupp Bastos. 2020. "Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis" Molecules 25, no. 9: 2060. https://doi.org/10.3390/molecules25092060
APA StyleCcana-Ccapatinta, G. V., Mejía, J. A. A., Tanimoto, M. H., Groppo, M., Carvalho, J. C. A. S. d., & Bastos, J. K. (2020). Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules, 25(9), 2060. https://doi.org/10.3390/molecules25092060