Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling
Abstract
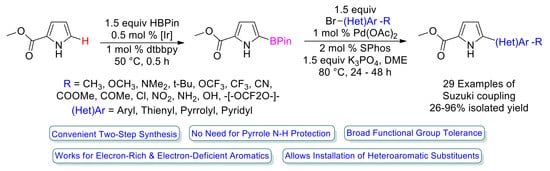
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations and Starting Materials
3.2. Suzuki Coupling
3.2.1. General Suzuki Procedure A Employing Pd(OAc)2 and 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (SPhos)
3.2.2. General Suzuki Procedure B Employing Palladium Tetrakistriphenylphosphine Pd(PPh3)4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domagala, A.; Jarosz, T.; Lapkowski, M. Living on pyrrolic foundations – Advances in natural and artificial bioactive pyrrole derivatives. Eur. J. Med. Chem. 2015, 100, 176–187. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Fukuda, T.; Umeki, T.; Tokushima, K.; Xiang, G.; Yoshida, Y.; Ishibashi, F.; Oku, Y.; Nishiya, N.; Uehara, Y.; Iwao, M. Design, synthesis, and evaluation of A-ring-modified lamellarin N analogues as noncovalent inhibitors of the EGFR T790M/L858R mutant. Bioorg. Med. Chem. 2017, 25, 6563–6580. [Google Scholar] [CrossRef] [Green Version]
- Lade, D.M.; Pawar, A.B.; Mainkar, P.S.; Chandrasekhar, S. Total Synthesis of Lamellarin D Trimethyl Ether, Lamellarin, D., and Lamellarin, H.J. Org. Chem. 2017, 82, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Imbri, D.; Tauber, J.; Opatz, T. Synthetic Approaches to the Lamellarins—A Comprehensive Review. Mar. Drugs 2014, 12, 6142–6177. [Google Scholar] [CrossRef] [Green Version]
- Dialer, C.; Imbri, D.; Hansen, S.P.; Opatz, T. Synthesis of Lamellarin D Trimethyl Ether and Lamellarin H via 6π-Electrocyclization. J. Org. Chem. 2015, 80, 11605–11610. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, R.S.Z.; Lansdell, T.A.; Tepe, J.J. Synthesis and evaluation of debromohymenialdisine-derived Chk2 inhibitors. Bioorg. Med. Chem. 2012, 20, 1475–1481. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Saleem, R.S.Z.; Luderer, M.J.; Hovde, S.; Henry, R.W.; Tepe, J.J. Radioprotection by Hymenialdisine-Derived Checkpoint Kinase 2 Inhibitors. ACS Chem. Biol. 2012, 7, 172–184. [Google Scholar] [CrossRef]
- Curreli, F.; Belov, D.S.; Kwon, Y.D.; Ramesh, R.; Furimsky, A.M.; O’Loughlin, K.; Byrge, P.C.; Iyer, L.V.; Mirsalis, J.C.; Kurkin, A.V.; et al. Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur. J. Med. Chem. 2018, 154, 367–391. [Google Scholar] [CrossRef]
- Curreli, F.; Kwon, Y.D.; Belov, D.S.; Ramesh, R.R.; Kurkin, A.V.; Altieri, A.; Kwong, P.D.; Debnath, A.K. Synthesis, Antiviral Potency, in Vitro ADMET, and X-ray Structure of Potent CD4 Mimics as Entry Inhibitors That Target the Phe43 Cavity of HIV-1 gp120. J. Med. Chem. 2017, 60, 3124–3153. [Google Scholar] [CrossRef] [PubMed]
- Curreli, F.; Belov, D.S.; Ramesh, R.R.; Patel, N.; Altieri, A.; Kurkin, A.V.; Debnath, A.K. Design, synthesis and evaluation of small molecule CD4-mimics as entry inhibitors possessing broad spectrum anti-HIV-1 activity. Bioorg. Med. Chem. 2016, 24, 5988–6003. [Google Scholar] [CrossRef] [Green Version]
- Curreli, F.; Kwon, Y.D.; Zhang, H.; Scacalossi, D.; Belov, D.S.; Tikhonov, A.A.; Andreev, I.A.; Altieri, A.; Kurkin, A.V.; Kwong, P.D.; et al. Structure-Based Design of a Small Molecule CD4-Antagonist with Broad Spectrum Anti-HIV-1 Activity. J. Med. Chem. 2015, 58, 6909–6927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, G.; Loriga, G.; Murineddu, G.; Grella, G.; Mura, M.; Vargiu, L.; Murgioni, C.; La Colla, P. Synthesis and Anti-HIV-1 Activity of New Delavirdine Analogues Carrying Arylpyrrole Moieties. Chem. Pharm. Bull. 2001, 49, 1406–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryavtsev, K.V.; Bentley, M.L.; McCafferty, D.G. Probing of the cis-5-phenyl proline scaffold as a platform for the synthesis of mechanism-based inhibitors of the Staphylococcus aureus sortase SrtA isoform. Bioorg. Med. Chem. 2009, 17, 2886–2893. [Google Scholar] [CrossRef] [Green Version]
- Banwell, M.G.; Hamel, E.; Hockless, D.C.R.; Verdier-Pinard, P.; Willis, A.C.; Wong, D.J. 4,5-Diaryl-1H-pyrrole-2-carboxylates as combretastatin A-4/lamellarin T hybrids: Synthesis and evaluation as anti-mitotic and cytotoxic agents. Bioorg. Med. Chem. 2006, 14, 4627–4638. [Google Scholar] [CrossRef]
- Galenko, E.E.; Galenko, A.V.; Novikov, M.S.; Khlebnikov, A.F.; Kudryavtsev, I.V.; Terpilowski, M.A.; Serebriakova, M.K.; Trulioff, A.S.; Goncharov, N.V. 4-Diazo and 4-(Triaz-1-en-1-yl)-1H-pyrrole-2-carboxylates as Agents Inducing Apoptosis. ChemistrySelect 2017, 2, 7508–7513. [Google Scholar] [CrossRef]
- Galenko, E.E.; Galenko, A.V.; Khlebnikov, A.F.; Novikov, M.S.; Shakirova, J.R. Synthesis and Intramolecular Azo Coupling of 4-Diazopyrrole-2-carboxylates: Selective Approach to Benzo and Hetero [c]-Fused 6H-Pyrrolo[3,4-c]pyridazine-5-carboxylates. J. Org. Chem. 2016, 81, 8495–8507. [Google Scholar] [CrossRef]
- Killoran, J.; Gallagher, J.F.; Murphy, P.V.; O’Shea, D.F. A study of the effects of subunit pre-orientation for diarylpyrrole esters; design of new aryl-heteroaryl fluorescent sensors. New J. Chem. 2005, 29, 1258–1265. [Google Scholar] [CrossRef]
- Granda, J.M.; Staszewska-Krajewska, O.; Jurczak, J. Bispyrrolylbenzene Anion Receptor: From Supramolecular Switch to Molecular Logic Gate. Chem. Eur. J. 2014, 20, 12790–12795. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.; Lammer, A.D.; Chi, X.; Brewster, J.T.; Lynch, V.M.; Li, H.; Zhang, Z.; Sessler, J.L. Self-Assembled Pyridine-Dipyrrolate Cages. J. Am. Chem. Soc. 2016, 138, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Chaolu, E.; Satoshi, H.; Jun-ichiro, S. One-Handed Single Helicates of Dinickel(II) Benzenehexapyrrole-α,ω-diimine with an Amine Chiral Source. Chem. Eur. J. 2015, 21, 239–246. [Google Scholar]
- Setsune, J.-i.; Kawama, M.; Nishinaka, T. Helical binuclear CoII complexes of pyriporphyrin analogue for sensing homochiral carboxylic acids. Tetrahedron Lett. 2011, 52, 1773–1777. [Google Scholar] [CrossRef]
- Boukou-Poba, J.P.; Farnier, M.; Guilard, R. A general method for the synthesis of 2-arylpyrroles. Tetrahedron Lett. 1979, 20, 1717–1720. [Google Scholar] [CrossRef]
- Ezquerra, J.; Pedregal, C.; Rubio, A.; Valenciano, J.; Navio, J.L.G.; Alvarez-Builla, J.; Vaquero, J.J. General method for the synthesis of 5-arylpyrrole-2-carboxylic acids. Tetrahedron Lett. 1993, 34, 6317–6320. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, M.-J.R.P.; Begouin, A.; Pereira, G.; Ferreira, P.M.T. New synthesis of methyl 5-aryl or heteroaryl pyrrole-2-carboxylates by a tandem Sonogashira coupling/5-endo-dig-cyclization from β-iododehydroamino acid methyl esters and terminal alkynes. Tetrahedron 2008, 64, 10714–10720. [Google Scholar] [CrossRef]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef]
- Cheng, B.-Y.; Wang, Y.-N.; Li, T.-R.; Lu, L.-Q.; Xiao, W.-J. Synthesis of Polysubstituted Pyrroles through a Formal [4 + 1] Cycloaddition/E1cb Elimination/Aromatization Sequence of Sulfur Ylides and α,β-Unsaturated Imines. J. Org. Chem. 2017, 82, 12134–12140. [Google Scholar] [CrossRef]
- Ngwerume, S.; Lewis, W.; Camp, J.E. Development of a Gold-Multifaceted Catalysis Approach to the Synthesis of Highly Substituted Pyrroles: Mechanistic Insights via Huisgen Cycloaddition Studies. J. Org. Chem. 2013, 78, 920–934. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Luo, X.; Han, D.-M.; Deng, W.-P. Direct synthesis of pyrroles via 1,3-dipolar cycloaddition of azomethine ylides with ynones. New J. Chem. 2013, 37, 1742–1745. [Google Scholar] [CrossRef]
- Kudryavtsev, K.V.; Ivantcova, P.M.; Churakov, A.V.; Vasin, V.A. Phenyl α-bromovinyl sulfone in cycloadditions with azomethine ylides: An unexpected facile aromatization of the cycloadducts into pyrroles. Tetrahedron Lett. 2012, 53, 4300–4303. [Google Scholar] [CrossRef]
- Lade, D.M.; Pawar, A.B. Cp*Co(iii)-catalyzed vinylic C-H bond activation under mild conditions: Expedient pyrrole synthesis via (3 + 2) annulation of enamides and alkynes. Org. Chem. Front. 2016, 3, 836–840. [Google Scholar] [CrossRef]
- Imbri, D.; Netz, N.; Kucukdisli, M.; Kammer, L.M.; Jung, P.; Kretzschmann, A.; Opatz, T. One-Pot Synthesis of Pyrrole-2-carboxylates and -carboxamides via an Electrocyclization/Oxidation Sequence. J. Org. Chem. 2014, 79, 11750–11758. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, A.; Robles-Machín, R.; Adrio, J.; Carretero, J.C. Oligopyrrole Synthesis by 1,3-Dipolar Cycloaddition of Azomethine Ylides with Bissulfonyl Ethylenes. Angew. Chem. Int. Ed. 2007, 46, 9261–9264. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.-M.; Li, H.-L.; He, F.-S.; Luo, X.; Deng, W.-P. Regioselective Iodine-Catalyzed Construction of Polysubstituted Pyrroles from Allenes and Enamines. J. Org. Chem. 2016, 81, 8653–8658. [Google Scholar] [CrossRef]
- Galenko, E.E.; Bodunov, V.A.; Galenko, A.V.; Novikov, M.S.; Khlebnikov, A.F. Fe(II)-Catalyzed Isomerization of 4-Vinylisoxazoles into Pyrroles. J. Org. Chem. 2017, 82, 8568–8579. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Stengel, T. Grubbs and Wilkinson catalyzed reactions of 2-phenyl-3-vinyl substituted 2H-azirines. Arkivoc 2004, 2005, 21–32. [Google Scholar]
- Farney, E.P.; Yoon, T.P. Visible-Light Sensitization of Vinyl Azides by Transition-Metal Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 793–797. [Google Scholar] [CrossRef]
- Dong, H.; Shen, M.; Redford, J.E.; Stokes, B.J.; Pumphrey, A.L.; Driver, T.G. Transition Metal-Catalyzed Synthesis of Pyrroles from Dienyl Azides. Org. Lett. 2007, 9, 5191–5194. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ji, C.L.; Hong, X.; Szostak, M. Palladium-Catalyzed Decarbonylative Borylation of Carboxylic Acids: Tuning Reaction Selectivity by Computation. Angew. Chem. Int. Ed. 2018, 57, 16721–16726. [Google Scholar] [CrossRef]
- Akira, S.; Yasunori, Y. Cross-coupling Reactions of Organoboranes: An Easy Method for C–C Bonding. Chem. Lett. 2011, 40, 894–901. [Google Scholar]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maiss, J.; Mohy El Dine, T.; Lu, C.-S.; Karamé, I.; Kanj, A.; Polychronopoulou, K.; Shaya, J. Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3)–C(sp2)) Suzuki-Miyaura Cross-Couplings. Catalysts 2020, 10, 296–320. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Szostak, M. Decarbonylative Borylation of Amides by Palladium Catalysis. ACS Omega 2019, 4, 4901–4907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martina, S.; Enkelmann, V.; Wegner, G.; Schlüter, A.-D. N-Protected Pyrrole Derivatives Substituted for Metal-Catalyzed Cross-Coupling Reactions. Synthesis 1991, 1991, 613–615. [Google Scholar] [CrossRef]
- Laha, J.K.; Sharma, S.; Bhimpuria, R.A.; Dayal, N.; Dubey, G.; Bharatam, P.V. Integration of oxidative arylation with sulfonyl migration: One-pot tandem synthesis of densely functionalized (NH)-pyrroles. New J. Chem. 2017, 41, 8791–8803. [Google Scholar] [CrossRef]
- Yiğit, B.; Gürbüz, N.; Yiğit, M.; Dağdeviren, Z.; Özdemir, İ. Palladium(II) N-heterocyclic carbene complexes as catalysts for the direct arylation of pyrrole derivatives with aryl chlorides. Inorg. Chim. Acta 2017, 465, 44–49. [Google Scholar] [CrossRef]
- Laha, J.K.; Bhimpuria, R.A.; Prajapati, D.V.; Dayal, N.; Sharma, S. Palladium-catalyzed regioselective C-2 arylation of 7-azaindoles, indoles, and pyrroles with arenes. Chem. Commun. 2016, 52, 4329–4332. [Google Scholar] [CrossRef]
- Carina, S.; Karthik, D.; Andreas, O.; Gates, P.J.; Pilarski, L.T. Ru-Catalysed C-H Arylation of Indoles and Pyrroles with Boronic Acids: Scope and Mechanistic Studies. Chem. Eur. J. 2015, 21, 5380–5386. [Google Scholar]
- Wang, L.; Li, Z.; Qu, X.; Peng, W. Highly Efficient Synthesis of Arylpyrrole Derivatives via Rh(III)-Catalyzed Direct C-H Arylation with Aryl Boronic Acids. Chin. J. Chem. 2015, 33, 1015–1018. [Google Scholar] [CrossRef]
- Pla, D.; Marchal, A.; Olsen, C.A.; Albericio, F.; Álvarez, M. Modular Total Synthesis of Lamellarin, D.J. Org. Chem. 2005, 70, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Belov, D.S.; Ivanov, V.N.; Curreli, F.; Kurkin, A.V.; Altieri, A.; Debnath, A.K. Synthesis of 5-Arylpyrrole-2-carboxylic Acids as Key Intermediates for NBD Series HIV-1 Entry Inhibitors. Synthesis 2017, 49, 3692–3699. [Google Scholar]
- Hodge, P.; Rickards, R.W. 72. The halogenation of methyl pyrrole-2-carboxylate and of some related pyrroles. J. Chem. Soc. 1965, 459–470. [Google Scholar] [CrossRef]
- Anderson, H.J.; Lee, S.-F. Pyrrole Chemistry: IV. The Preparation and some reactions of brominated pyrrole derivatives. Can. J. Chem. 1965, 43, 409–414. [Google Scholar] [CrossRef]
- Chen, W.; Cava, M.P. Convenient synthetic equivalents of 2-lithiopyrrole and 2,5-dilithiopyrrole. Tetrahedron Lett. 1987, 28, 6025–6026. [Google Scholar] [CrossRef]
- Komatsubara, M.; Umeki, T.; Fukuda, T.; Iwao, M. Modular Synthesis of Lamellarins via Regioselective Assembly of 3,4,5-Differentially Arylated Pyrrole-2-carboxylates. J. Org. Chem. 2014, 79, 529–537. [Google Scholar] [CrossRef]
- Urbano, M.; Guerrero, M.; Zhao, J.; Velaparthi, S.; Schaeffer, M.-T.; Brown, S.; Rosen, H.; Roberts, E. SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P4) receptor. Bioorg. Med. Chem. Lett. 2011, 21, 5470–5474. [Google Scholar] [CrossRef] [Green Version]
- Setsune, J.-i.; Toda, M.; Watanabe, K.; Panda, P.K.; Yoshida, T. Synthesis of bis(pyrrol-2-yl)arenes by Pd-catalyzed cross coupling. Tetrahedron Lett. 2006, 47, 7541–7544. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-Y.; Tse, M.K.; Holmes, D.; Maleczka, R.E., Jr.; Smith, M.R., III. Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C-H Bonds. Science 2002, 295, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N.R.; Hartwig, J.F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C−H Activation for the Construction of C−B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Recent advances in catalytic C−H borylation reactions. Tetrahedron 2017, 73, 7123–7157. [Google Scholar] [CrossRef]
- Takagi, J.; Sato, K.; Hartwig, J.F.; Ishiyama, T.; Miyaura, N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: Regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 2002, 43, 5649–5651. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nobuta, Y.; Hartwig, J.F.; Miyaura, N. Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent. Chem. Commun. 2003, 23, 2924–2925. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R.; Yeung, K. The synthesis of novel pyrrololactams and their boronate ester derivatives. Tetrahedron Lett. 2016, 57, 5812–5814. [Google Scholar] [CrossRef]
- Paul, S.; Chotana, G.A.; Holmes, D.; Reichle, R.C.; Maleczka, R.E., Jr.; Smith, M.R., III. Ir-Catalyzed Functionalization of 2-Substituted Indoles at the 7-Position: Nitrogen-Directed Aromatic Borylation. J. Am. Chem. Soc. 2006, 128, 15552–15553. [Google Scholar] [CrossRef]
- Robbins, D.W.; Boebel, T.A.; Hartwig, J.F. Iridium-Catalyzed, Silyl-Directed Borylation of Nitrogen-Containing Heterocycles. J. Am. Chem. Soc. 2010, 132, 4068–4069. [Google Scholar] [CrossRef]
- Shen, F.; Tyagarajan, S.; Perera, D.; Krska, S.W.; Maligres, P.E.; Smith, M.R., III; Maleczka, R.E., Jr. Bismuth Acetate as a Catalyst for the Sequential Protodeboronation of Di- and Triborylated Indoles. Org. Lett. 2016, 18, 1554–1557. [Google Scholar] [CrossRef] [Green Version]
- Eastabrook, A.S.; Sperry, J. Synthetic Access to 3,5,7-Trisubstituted Indoles Enabled by Iridium Catalyzed C–H Borylation. Synthesis 2017, 49, 4731–4737. [Google Scholar]
- Chotana, G.A.; Kallepalli, V.A.; Maleczka, R.E., Jr.; Smith, M.R., III. Iridium-catalyzed borylation of thiophenes: Versatile, synthetic elaboration founded on selective C–H functionalization. Tetrahedron 2008, 64, 6103–6114. [Google Scholar] [CrossRef] [Green Version]
- Sadler, S.A.; Tajuddin, H.; Mkhalid, I.A.I.; Batsanov, A.S.; Albesa-Jove, D.; Cheung, M.S.; Maxwell, A.C.; Shukla, L.; Roberts, B.; Blakemore, D.C.; et al. Iridium-catalyzed C-H borylation of pyridines. Org. Biomol. Chem. 2014, 12, 7318–7327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batool, F.; Emwas, A.-H.; Gao, X.; Munawar, M.A.; Chotana, G.A. Synthesis and Suzuki Cross-Coupling Reactions of 2,6-Bis(trifluoromethyl)pyridine-4-boronic Acid Pinacol Ester. Synthesis 2017, 49, 1327–1334. [Google Scholar]
- Yang, L.; Semba, K.; Nakao, Y. para-Selective C−H Borylation of (Hetero)Arenes by Cooperative Iridium/Aluminum Catalysis. Angew. Chem. Int. Ed. 2017, 56, 4853–4857. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.A.; Hartwig, J.F. Iridium-Catalyzed C–H Borylation of Heteroarenes: Scope, Regioselectivity, Application to Late-Stage Functionalization, and Mechanism. J. Am. Chem. Soc. 2014, 136, 4287–4299. [Google Scholar] [CrossRef]
- Tse, M.K.; Cho, J.-Y.; Smith, M.R., III. Regioselective Aromatic Borylation in an Inert Solvent. Org. Lett. 2001, 3, 2831–2833. [Google Scholar]
- Kallepalli, V.A.; Shi, F.; Paul, S.; Onyeozili, E.N.; Maleczka, R.E., Jr.; Smith, M.R., III. Boc Groups as Protectors and Directors for Ir-Catalyzed C−H Borylation of Heterocycles. J. Org. Chem. 2009, 74, 9199–9201. [Google Scholar] [CrossRef] [Green Version]
- Swartz, D.L., II; Staples, R.J.; Odom, A.L. Synthesis and hydroamination catalysis with 3-aryl substituted pyrrolyl and dipyrrolylmethane titanium(iv) complexes. Dalton Trans. 2011, 40, 7762–7768. [Google Scholar] [CrossRef]
- Robbins, D.W.; Hartwig, J.F. A C–H Borylation Approach to Suzuki–Miyaura Coupling of Typically Unstable 2–Heteroaryl and Polyfluorophenyl Boronates. Org. Lett. 2012, 14, 4266–4269. [Google Scholar] [CrossRef]
- Asghar, S.; Shahzadi, T.; Alazmi, M.; Gao, X.; Emwas, A.-H.; Saleem, R.S.Z.; Batool, F.; Chotana, G.A. Iridium-Catalyzed Regioselective Borylation of Substituted Biaryls. Synthesis 2018, 50, 2211–2220. [Google Scholar]
- Batool, F.; Parveen, S.; Emwas, A.-H.; Sioud, S.; Gao, X.; Munawar, M.A.; Chotana, G.A. Synthesis of Fluoroalkoxy Substituted Arylboronic Esters by Iridium-Catalyzed Aromatic C–H Borylation. Org. Lett. 2015, 17, 4256–4259. [Google Scholar] [CrossRef]
- Shahzadi, T.; Saleem, R.S.Z.; Chotana, G.A. Facile Synthesis of Halogen Decorated para-/meta-Hydroxy benzoates by Iridium-Catalyzed Borylation and Oxidation. Synthesis 2018, 50, 4336–4342. [Google Scholar]
- Ikram, H.M.; Rasool, N.; Ahmad, G.; Chotana, G.A.; Musharraf, S.G.; Zubarir, M.; Rana, U.A.; Zia-ul-Haq, M.; Jaafar, H.Z. Selective C-Arylation of 2,5-Dibromo-3-hexylthiophene via Suzuki Cross Coupling Reaction and Their Pharmacological Aspects. Molecules 2015, 20, 5202–5214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikram, H.M.; Rasool, N.; Zubair, M.; Khan, K.M.; Chotana, G.A.; Akhtar, M.N.; Abu, N.; Alitheen, N.B.; Elgorban, A.M.; Rana, U.A. Efficient Double Suzuki Cross-Coupling Reactions of 2,5-Dibromo-3-hexylthiophene: Anti-Tumor, Haemolytic, Anti-Thrombolytic and Biofilm Inhibition Studies. Molecules 2016, 21, 977–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, F.; Zakir, H.; Asghar, S.; Abbas, G.; Riaz, M. Malus domestica Mediated Synthesis of Palladium Nanoparticles and Investigation of Their Catalytic Activity Towards the Suzuki Coupling Reactions. Nanosci. Nanotechnol. Lett. 2018, 10, 373–377. [Google Scholar] [CrossRef]
- Miller, S.L.; Chotana, G.A.; Fritz, J.A.; Chattopadhyay, B.; Maleczka, R.E., Jr.; Smith, M.R., III. C-H Borylation Catalysts that Distinguish between Similarly Sized Substituents like Fluorine and Hydrogen. Org. Lett. 2019, 21, 6388–6392. [Google Scholar] [CrossRef]
- Chotana, G.A.; Montero Bastidas, J.R.; Miller, S.L.; Smith, M.R., III.; Maleczka, R.E., Jr. One-Pot Iridium-Catalyzed C–H Borylation/Sonogashira Cross-Coupling: Access to Borylated Aryl Alkynes. Molecules 2020, 25, 1754–1766. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, T.; Takagi, J.; Yonekawa, Y.; Hartwig, J.F.; Miyaura, N. Iridium-Catalyzed Direct Borylation of Five-Membered Heteroarenes by Bis(pinacolato)diboron: Regioselective, Stoichiometric, and Room Temperature Reactions. Adv. Synth. Catal. 2003, 345, 1103–1106. [Google Scholar] [CrossRef]
- Billingsley, K.L.; Anderson, K.W.; Buchwald, S.L. A Highly Active Catalyst for Suzuki–Miyaura Cross-Coupling Reactions of Heteroaryl Compounds. Angew. Chem. Int. Ed. 2006, 45, 3484–3488. [Google Scholar] [CrossRef]
- Rieth, R.D.; Mankad, N.P.; Calimano, E.; Sadighi, J.P. Palladium-Catalyzed Cross-Coupling of Pyrrole Anions with Aryl Chlorides, Bromides, and Iodides. Org. Lett. 2004, 6, 3981–3983. [Google Scholar] [CrossRef]
- Farnier, M.; Soth, S.; Fournari, P. Recherches en série hétérocyclique. XXVIII. Synthèse de bipyrroles. Can. J. Chem. 1976, 54, 1083–1086. [Google Scholar]
- Castro, A.J.; Giannini, D.D.; Greenlee, W.F. Synthesis of a 2,3’-bipyrrole. Denitrosation in the Knorr pyrrole synthesis. J. Org. Chem. 1970, 35, 2815–2816. [Google Scholar]
- Dohi, T.; Morimoto, K.; Maruyama, A.; Kita, Y. Direct Synthesis of Bipyrroles Using Phenyliodine Bis(trifluoroacetate) with Bromotrimethylsilane. Org. Lett. 2006, 8, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, S.; Ann, N.-u.-; Fatima, S.; Emwas, A.-H.; Alazmi, M.; Gao, X.; Ibrar, M.; Zaib Saleem, R.S.; Chotana, G.A. Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules 2020, 25, 2106. https://doi.org/10.3390/molecules25092106
Kanwal S, Ann N-u-, Fatima S, Emwas A-H, Alazmi M, Gao X, Ibrar M, Zaib Saleem RS, Chotana GA. Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules. 2020; 25(9):2106. https://doi.org/10.3390/molecules25092106
Chicago/Turabian StyleKanwal, Saba, Noor-ul- Ann, Saman Fatima, Abdul-Hamid Emwas, Meshari Alazmi, Xin Gao, Maha Ibrar, Rahman Shah Zaib Saleem, and Ghayoor Abbas Chotana. 2020. "Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling" Molecules 25, no. 9: 2106. https://doi.org/10.3390/molecules25092106
APA StyleKanwal, S., Ann, N. -u. -, Fatima, S., Emwas, A. -H., Alazmi, M., Gao, X., Ibrar, M., Zaib Saleem, R. S., & Chotana, G. A. (2020). Facile Synthesis of NH-Free 5-(Hetero)Aryl-Pyrrole-2-Carboxylates by Catalytic C–H Borylation and Suzuki Coupling. Molecules, 25(9), 2106. https://doi.org/10.3390/molecules25092106