New Oxindole-Bridged Acceptors for Organic Sensitizers: Substitution and Performance Studies in Dye-Sensitized Solar Cells
Abstract
:1. Introduction
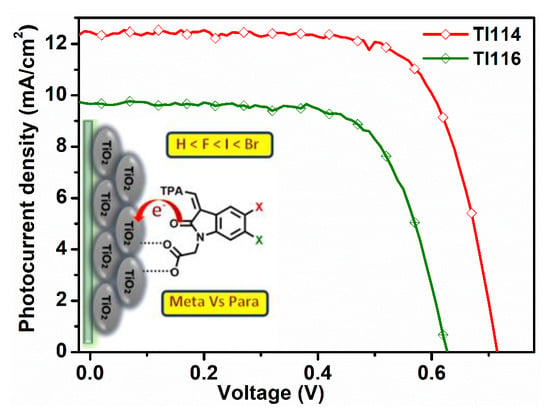
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grätzel, M. Mesoscopic solar cells for electricity and hydrogen production from sunlight. Chem. Lett. 2005, 34, 8–13. [Google Scholar] [CrossRef]
- Yun, S.; Lund, P.D.; Hinsch, A. Stability assessment of alternative platinum free counter electrodes for dye-sensitized solar cells. Energy Environ. Sci. 2015, 8, 3495–3514. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef] [PubMed]
- Sugathan, V.; John, E.; Sudhakar, K.J.R.; Reviews, S.E. Recent improvements in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Docampo, P.; Guldin, S.; Leijtens, T.; Noel, N.K.; Steiner, U.; Snaith, H.J. Lessons Learned: From Dye-Sensitized Solar Cells to All-Solid-State Hybrid Devices. Adv. Mater. 2014, 26, 4013–4030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Numata, Y.; Han, L. Highly efficient dye-sensitized solar cells: Progress and future challenges. Energy Environ. Sci. 2013, 6, 1443–1464. [Google Scholar] [CrossRef]
- Grätzel, C.; Zakeeruddin, S.M. Recent trends in mesoscopic solar cells based on molecular and nanopigment light harvesters. Mater. Today 2013, 16, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Hardin, B.E.; Snaith, H.J.; McGehee, M.D. The renaissance of dye-sensitized solar cells. Nat. Photon. 2012, 6, 162–169. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Robertson, N. Optimizing Dyes for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2006, 45, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Grätzel, M. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 9, Chapter 16. [Google Scholar]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Maddah, H.A.; Berry, V.; Behura, S.K. Biomolecular photosensitizers for dye-sensitized solar cells: Recent developments and critical insights. Renew. Sustain. Energy Rev. 2020, 121, 109678. [Google Scholar] [CrossRef]
- Boschloo, G. Improving the performance of dye-sensitized solar cells. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Yin, J.-F.; Velayudham, M.; Bhattacharya, D.; Lin, H.-C.; Lu, K.-L. Structure optimization of ruthenium photosensitizers for efficient dye-sensitized solar cells—A goal toward a “bright” future. Coord. Chem. Rev. 2012, 256, 3008–3035. [Google Scholar] [CrossRef]
- Robson, K.C.D.; Bomben, P.G.; Berlinguette, C.P. Cycloruthenated sensitizers: Improving the dye-sensitized solar cell with classical inorganic chemistry principles. Dalton Trans. 2012, 41, 7814–7829. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Müller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2’-bipyridyl-4,4’-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Splivallo, R.; Liska, P.; Comte, P.; Grätzel, M. A swift dye uptake procedure for dye sensitized solar cells. Chem. Commun. 2003, 9, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M.J.J.o.P.; Chemistry, P.A. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, C.; Ashraful, I.; Yuki, W.; Ryoichi, K.; Naoki, K.; Liyuan, H. Dye-Sensitized Solar Cells with Conversion Efficiency of 11.1%. Jpn. J. Appl. Phys. Part 1 2006, 45, L638. [Google Scholar]
- Wang, S.-W.; Chou, C.-C.; Hu, F.-C.; Wu, K.-L.; Chi, Y.; Clifford, J.N.; Palomares, E.; Liu, S.-H.; Chou, P.-T.; Wei, T.-C.; et al. Panchromatic Ru(ii) sensitizers bearing single thiocyanate for high efficiency dye sensitized solar cells. J. Mater. Chem. A 2014, 2, 17618–17627. [Google Scholar] [CrossRef]
- Jia, H.-L.; Li, S.-S.; Gong, B.-Q.; Gu, L.; Bao, Z.-L.; Guan, M.-Y. Efficient cosensitization of new organic dyes containing bipyridine anchors with porphyrins for dye-sensitized solar cells. Sustain. Energy Fuels 2020, 4, 347–353. [Google Scholar] [CrossRef]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalton Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef]
- Yella, A.; Mai, C.-L.; Zakeeruddin, S.M.; Chang, S.-N.; Hsieh, C.-H.; Yeh, C.-Y.; Grätzel, M. Molecular Engineering of Push–Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.-Y.; Diau, E.W.-G.; Grätzel, M. Highly Efficient Mesoscopic Dye-Sensitized Solar Cells Based on Donor–Acceptor-Substituted Porphyrins. Angew. Chem. Int. Ed. 2010, 49, 6646–6649. [Google Scholar] [CrossRef] [PubMed]
- Collavini, S.; Völker, S.F.; Delgado, J.L. Understanding the Outstanding Power Conversion Efficiency of Perovskite-Based Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, L.; Mei, A.; Li, X.; Han, H. Beyond Efficiency: The Challenge of Stability in Mesoscopic Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1501066. [Google Scholar] [CrossRef]
- Yen, Y.-S.; Chou, H.-H.; Chen, Y.-C.; Hsu, C.-Y.; Lin, J.T. Recent developments in molecule-based organic materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 8734–8747. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D-[small pi]-A to D-A-[small pi]-A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Kanaparthi, R.K.; Kandhadi, J.; Giribabu, L. Metal-free organic dyes for dye-sensitized solar cells: Recent advances. Tetrahedron 2012, 68, 8383–8393. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Molecular Designs and Syntheses of Organic Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2009, 18, 2903–2934. [Google Scholar] [CrossRef]
- Cong, J.; Yang, X.; Liu, J.; Zhao, J.; Hao, Y.; Wang, Y.; Sun, L. Nitro group as a new anchoring group for organic dyes in dye-sensitized solar cells. Chem. Commun. 2012, 48, 6663–6665. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.R.; Ward, M.D.; Nazeeruddin, M.K.; Grätzel, M. Catechol as an efficient anchoring group for attachment of ruthenium-polypyridine photosensitisers to solar cells based on nanocrystalline TiO2 films. New J. Chem. 2000, 24, 651–652. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.-H.; Grätzel, M. Acid−Base Equilibria of (2,2‘-Bipyridyl-4,4‘-dicarboxylic acid)ruthenium(II) Complexes and the Effect of Protonation on Charge-Transfer Sensitization of Nanocrystalline Titania. Inorg. Chem. 1999, 38, 6298–6305. [Google Scholar] [CrossRef]
- Numata, Y.; Ashraful, I.; Shirai, Y.; Han, L. Preparation of donor-acceptor type organic dyes bearing various electron-withdrawing groups for dye-sensitized solar cell application. Chem. Commun. 2011, 47, 6159–6161. [Google Scholar] [CrossRef]
- Delcamp, J.H.; Yella, A.; Nazeeruddin, M.K.; Grätzel, M. Modulating dye E(S+/S*) with efficient heterocyclic nitrogen containing acceptors for DSCs. Chem. Commun. 2012, 48, 2295–2297. [Google Scholar] [CrossRef]
- Hong, J.; Lai, H.; Liu, Y.; Yuan, C.; Li, Y.; Liu, P.; Fang, Q. New organic dyes containing E- or Z-trifluoromethyl acrylic acid as the electron acceptors for dye-sensitized solar cell applications: An investigation of the effect of molecular configuration on the power conversion efficiency of the cells. RSC Adv. 2013, 3, 1069–1072. [Google Scholar] [CrossRef]
- Li, T.-Y.; Su, C.; Akula, S.B.; Sun, W.-G.; Chien, H.-M.; Li, W.-R. New Pyridinium Ylide Dyes for Dye Sensitized Solar Cell Applications. Org. Lett. 2016, 18, 3386–3389. [Google Scholar] [CrossRef]
- Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Sun, L.; Tran, N.; Tang, F.; App, H.; Hirth, P.; McMahon, G.; Tang, C. Synthesis and Biological Evaluations of 3-Substituted Indolin-2-ones: A Novel Class of Tyrosine Kinase Inhibitors That Exhibit Selectivity toward Particular Receptor Tyrosine Kinases. J. Med. Chem. 1998, 41, 2588–2603. [Google Scholar] [CrossRef]
- Marfat, A.; Robinson, R.P. Preparation of azaoxindole-1-carboxamides as antiinflammatories and analgesics. U.S. Patent 5,811,432 A, 22 September 1998. [Google Scholar]
- Robinson, R.R.; Donahue, K.M.; Son, P.S.; Wagy, S.D. Synthesis of substituted azaoxindoles for the preparation of aza-tenidap analogs. J. Heterocyclic Chem. 1996, 33, 287–293. [Google Scholar] [CrossRef]
- Tingare, Y.S.; Shen, M.-T.; Su, C.; Ho, S.-Y.; Tsai, S.-H.; Chen, B.-R.; Li, W.-R. Novel Oxindole Based Sensitizers: Synthesis and Application in Dye-Sensitized Solar Cells. Org. Lett. 2013, 15, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.J.C.; Parlane, F.G.L.; Swords, W.B.; Kellett, C.W.; Du, C.; Lam, B.; Dean, R.K.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Halogen Bonding Promotes Higher Dye-Sensitized Solar Cell Photovoltages. J. Am. Chem. Soc. 2016, 138, 10406–10409. [Google Scholar] [CrossRef] [PubMed]
- Yoder, N.C.; Kumar, K. Fluorinated amino acids in protein design and engineering. Chem. Soc. Rev. 2002, 31, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated organic materials for electronic and optoelectronic applications: The role of the fluorine atom. Chem. Commun. 2007, 10, 1003–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Chen, D.-Y.; Chen, C.-L.; Hsu, C.-W.; Hsu, H.-C.; Wu, K.-L.; Liu, S.-H.; Chou, P.-T.; Chi, Y. Donor-acceptor dyes with fluorine substituted phenylene spacer for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 1937–1945. [Google Scholar] [CrossRef]
- Bhim Raju, T.; Vaghasiya, J.V.; Afroz, M.A.; Soni, S.S.; Iyer, P.K. Design, synthesis and DSSC performance of o-fluorine substituted phenylene spacer sensitizers: Effect of TiO2 thickness variation. Phys. Chem. Chem. Phys. 2016, 18, 28485–28491. [Google Scholar] [CrossRef]
- Jie, J.; Xu, Q.; Yang, G.; Feng, Y.; Zhang, B. Porphyrin sensitizers involving a fluorine-substituted benzothiadiazole as auxiliary acceptor and thiophene as π bridge for use in dye-sensitized solar cells (DSSCs). Dyes Pigments 2020, 174, 107984. [Google Scholar] [CrossRef]
- Mao, J.; He, N.; Ning, Z.; Zhang, Q.; Guo, F.; Chen, L.; Wu, W.; Hua, J.; Tian, H. Stable Dyes Containing Double Acceptors without COOH as Anchors for Highly Efficient Dye-Sensitized Solar Cells. Angew. Chem. 2012, 124, 10011–10014. [Google Scholar] [CrossRef]
- Mann, J.R.; Gannon, M.K.; Fitzgibbons, T.C.; Detty, M.R.; Watson, D.F. Optimizing the Photocurrent Efficiency of Dye-Sensitized Solar Cells through the Controlled Aggregation of Chalcogenoxanthylium Dyes on Nanocrystalline Titania Films. J. Phys. Chem. C 2008, 112, 13057–13061. [Google Scholar] [CrossRef]
- Kuang, D.; Uchida, S.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. Organic Dye-Sensitized Ionic Liquid Based Solar Cells: Remarkable Enhancement in Performance through Molecular Design of Indoline Sensitizers. Angew. Chem. Int. Ed. 2008, 47, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 2007, 36, 3741–3743. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, X.; Tian, H.; Sun, L. Tetrahydroquinoline dyes with different spacers for organic dye-sensitized solar cells. J. Photochem. Photobiol. A 2007, 189, 295–300. [Google Scholar] [CrossRef]
- Hara, K.; Wang, Z.-S.; Sato, T.; Furube, A.; Katoh, R.; Sugihara, H.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Suga, S. Oligothiophene-Containing Coumarin Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 15476–15482. [Google Scholar] [CrossRef]
- Liu, W.-H.; Wu, I.C.; Lai, C.-H.; Lai, C.-H.; Chou, P.-T.; Li, Y.-T.; Chen, C.-L.; Hsu, Y.-Y.; Chi, Y. Simple organic molecules bearing a 3,4-ethylenedioxythiophene linker for efficient dye-sensitized solar cells. Chem. Commun. 2008, 41, 5152–5154. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available from the authors. |






| Dye | λabs/nm (ε/L mol−1 cm−1) | EHOMO[a] (eV) | ELUMO[b] (eV) | E0-0[b] (eV) |
|---|---|---|---|---|
| TI111 | 469 (3.7737) | 5.11 | 3.02 | 2.09 |
| TI112 | 480 (3.0562) | 5.11 | 3.05 | 2.06 |
| TI114 | 483 (3.8825) | 5.11 | 3.08 | 2.03 |
| TI115 | 483 (3.4712) | 5.12 | 3.09 | 2.03 |
| TI116 | 410 (1.7437) | 5.11 | 3.06 | 2.05 |
| Sensitizer | JSC (mA cm−2) | VOC (mV) | FF | η (%) |
|---|---|---|---|---|
| TI111 | 10.03 | 680 | 0.669 | 4.76 |
| TI112 | 11.32 | 690 | 0.695 | 5.43 |
| TI114 | 12.46 | 720 | 0.708 | 6.35 |
| TI115 | 11.97 | 680 | 0.714 | 5.81 |
| TI116 | 9.66 | 630 | 0.692 | 4.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tingare, Y.S.; Su, C.; Shen, M.-T.; Tsai, S.-H.; Ho, S.-Y.; Li, W.-R. New Oxindole-Bridged Acceptors for Organic Sensitizers: Substitution and Performance Studies in Dye-Sensitized Solar Cells. Molecules 2020, 25, 2159. https://doi.org/10.3390/molecules25092159
Tingare YS, Su C, Shen M-T, Tsai S-H, Ho S-Y, Li W-R. New Oxindole-Bridged Acceptors for Organic Sensitizers: Substitution and Performance Studies in Dye-Sensitized Solar Cells. Molecules. 2020; 25(9):2159. https://doi.org/10.3390/molecules25092159
Chicago/Turabian StyleTingare, Yogesh S., Chaochin Su, Ming-Tai Shen, Sheng-Han Tsai, Shih-Yu Ho, and Wen-Ren Li. 2020. "New Oxindole-Bridged Acceptors for Organic Sensitizers: Substitution and Performance Studies in Dye-Sensitized Solar Cells" Molecules 25, no. 9: 2159. https://doi.org/10.3390/molecules25092159
APA StyleTingare, Y. S., Su, C., Shen, M. -T., Tsai, S. -H., Ho, S. -Y., & Li, W. -R. (2020). New Oxindole-Bridged Acceptors for Organic Sensitizers: Substitution and Performance Studies in Dye-Sensitized Solar Cells. Molecules, 25(9), 2159. https://doi.org/10.3390/molecules25092159