Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers
Abstract
:1. Introduction
1.1. The Photoactive Materials of Choice-Structural Parameters and Molecular Design
1.2. The Device Architecture, Energetic Considerations, and Interface Engineering
1.3. The Donor/Acceptor Interface (D/A-I) – Morphological Properties and Nanoscale Evolution
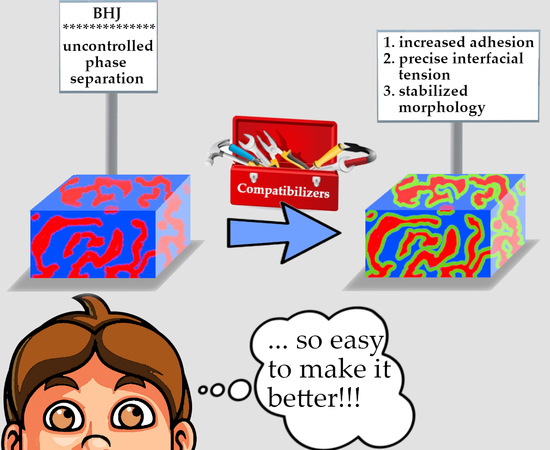
2. The Compatibilizers
2.1. Polymers
2.1.1. Thiophene-Containing Polymeric CBs
2.1.2. Thiophene-and Fullerene-Containing Polymeric CBs
2.1.3. Polymeric CBs without Thiophene and/or Fullerene-Fragments
2.2. Molecules
2.2.1. Aromatic-Core-Based Compatibilizers
2.2.2. Fulleroids
2.2.3. Non-Conjugated Molecules
3. Conclusion and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Cao, H.; He, W.; Mao, Y.; Lin, X.; Ishikawa, K.; Dickerson, J.H.; Hess, W.P. Recent progress in degradation and stabilization of organic solar cells. J. Power Sources 2014, 264, 168–183. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2017, 8, 1701791. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Chiappara, C.; Figà, V.; Di Marco, G.; Calogero, G.; Citro, I.; Scuto, A.; Lombardo, S.; Pignataro, B.G.; Principato, F. Investigation of recovery mechanisms in dye sensitized solar cells. Sol. Energy 2016, 127, 56–66. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Robbiano, V.; Paternò, G.M.; Cotella, G.F.; Fiore, T.; Dianetti, M.; Scopelliti, M.; Brunetti, F.; Pignataro, B.; Cacialli, F. Polystyrene Nanoparticle-Templated Hollow Titania Nanosphere Monolayers as Ordered Scaffolds. J. Mater. Chem. C 2018, 6, 2502–2508. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, G.; Cataldo, S.; Scopelliti, M.; Principato, F.; Chillura Martino, D.; Fiore, T.; Pignataro, B. Nonprecious Copper-Based Transparent Top Electrode via Seed Layer–Assisted Thermal Evaporation for High-Performance Semitransparent n-i-p Perovskite Solar Cells. Adv. Mater. Technol. 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Bredas, J.-L.; Durrant, J.R. Organic Photovoltaics. Acc. Chem. Res. 2009, 42, 1689–1690. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-W.; Lan, S.-C.; Wei, K.-H. Organic photovoltaics. Mater. Today 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Carlé, J.E.; Krebs, F.C. Technological status of organic photovoltaics (OPV). Sol. Energy Mater. Sol. Cells 2013, 119, 309–310. [Google Scholar] [CrossRef] [Green Version]
- Brabec, C.J.; Hauch, J.A.; Schilinsky, P.; Waldauf, C. Production Aspects of Organic Photovoltaics and Their Impact on the Commercialization of Devices. MRS Bull. 2005, 30, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.E.; Ginley, D.S.; Jabbour, G.E. Organic-Based Photovoltaics: Toward Low-Cost Power Generation. MRS Bull. 2005, 30, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Solution-processable polymeric solar cells: A review on materials, strategies and cell architectures to overcome 10%. Org. Electron. 2015, 19, 34–60. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhang, H.; Hou, J. Green-Solvent-Processable Organic Solar Cells. Mater. Today 2016, 19, 533–543. [Google Scholar] [CrossRef]
- Burgués-Ceballos, I.; Stella, M.; Lacharmoise, P.; Martínez-Ferrero, E. Towards Industrialization of Polymer Solar Cells: Material Processing for Upscaling. J. Mater. Chem. A 2014, 2, 17711–17722. [Google Scholar] [CrossRef]
- Wang, G.; Adil, M.A.; Zhang, J.; Wei, Z. Large-Area Organic Solar Cells: Material Requirements, Modular Designs, and Printing Methods. Adv. Mater. 2018, 31, 1805089. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Müllen, K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef]
- Li, C.-Z.; Yip, H.-L.; Jen, A. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161. [Google Scholar] [CrossRef]
- Qu, S.; Tian, H. Diketopyrrolopyrrole (DPP)-based materials for organic photovoltaics. Chem. Commun. 2012, 48, 3039. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhan, X. Non-fullerene acceptors for organic photovoltaics: An emerging horizon. Mater. Horiz. 2014, 1, 470. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Blanchard, P. Molecular Materials for Organic Photovoltaics: Small is Beautiful. Adv. Mater. 2014, 26, 3821–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zheng, Y.; Huang, J. Towards High Performance Organic Photovoltaic Cells: A Review of Recent Development in Organic Photovoltaics. Polymers 2014, 6, 2473–2509. [Google Scholar] [CrossRef] [Green Version]
- Hubler, A.; Trnovec, B.; Zillger, T.; Ali, M.; Wetzold, N.; Mingebach, M.; Wagenpfahl, A.; Deibel, C.; Dyakonov, V. Printed Paper Photovoltaic Cells. Adv. Energy Mater. 2011, 1, 1018–1022. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Arrabito, G.; Errico, V.; Zhang, Z.; Han, W.; Falconi, C. Nanotransducers on Printed Circuit Boards by Ra-tional Design of High-Density, Long, Thin and Untapered ZnO Nanowires. Nano Energy 2018, 46, 54–62. [Google Scholar] [CrossRef]
- Archet, F.; Yao, D.; Chambon, S.; Abbas, M.; D’Aléo, A.; Canard, G.; Ponce-Vargas, M.; Zaborova, E.; Le Guennic, B.; Wantz, G.; et al. Synthesis of Bioinspired Curcuminoid Small Molecules for Solution-Processed Organic Solar Cells with High Open-Circuit Voltage. ACS Energy Lett. 2017, 2, 1303–1307. [Google Scholar] [CrossRef]
- Poskela, A.; Miettunen, K.E.; Borghei, M.; Vapaavuori, J.; Greca, L.G.; Lehtonen, J.; Solin, K.; Ago, M.; Lund, P.D.; Rojas, O.J. Nanocellulose and Nanochitin Cryogels Improve the Efficiency of Dye Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 10257–10265. [Google Scholar] [CrossRef] [Green Version]
- Burke, D.J.; Lipomi, D.J. Green chemistry for organic solar cells. Energy Environ. Sci. 2013, 6, 2053. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Jacob, M.V.; Ostrikov, K. Sustainable Life Cycles of Natural-Precursor-Derived Nanocarbons. Chem. Rev. 2015, 116, 163–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Xu, L.; Li, J.; Sun, X.; Peng, H. Flexible solar cells based on carbon nanomaterials. Carbon 2018, 139, 1063–1073. [Google Scholar] [CrossRef]
- Leo, K. Organic photovoltaics. Nat. Rev. Mater. 2016, 1, 16056. [Google Scholar] [CrossRef]
- Levchenko, I.; Keidar, M.; Cantrell, J.; Wu, Y.-L.; Kuninaka, H.; Bazaka, K.; Xu, S. Explore space using swarms of tiny satellites. Nature 2018, 562, 185–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levchenko, I.; Bazaka, K.; Belmonte, T.; Keidar, M.; Xu, S. Advanced Materials for Next-Generation Space-craft. Adv. Mater. 2018, 30, 1802201. [Google Scholar] [CrossRef]
- Cardinaletti, I.; Vangerven, T.; Nagels, S.; Cornelissen, R.; Schreurs, D.; Hruby, J.; Vodnik, J.; Devisscher, D.; Kesters, J.; D’Haen, J.; et al. Organic and perovskite solar cells for space applications. Sol. Energy Mater. Sol. Cells 2018, 182, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, I.; Xu, S.; Teel, G.; Mariotti, D.; Walker, M.L.R.; Keidar, M. Recent progress and perspectives of space electric propulsion systems based on smart nanomaterials. Nat. Commun. 2018, 9, 879. [Google Scholar] [CrossRef]
- Hill, C.A. Satellite Battery Technology—A Tutorial and Overview. IEEE Aerosp. Electron. Syst. Mag. 2011, 26, 38–43. [Google Scholar] [CrossRef]
- Pelzer, K.M.; Darling, S.B. Charge generation in organic photovoltaics: A review of theory and computation. Mol. Syst. Des. Eng. 2016, 1, 10–24. [Google Scholar] [CrossRef]
- Braun, C.L. Electric field assisted dissociation of charge transfer states as a mechanism of photocarrier production. J. Chem. Phys. 1984, 80, 4157. [Google Scholar] [CrossRef]
- Liu, K.; Zeng, B.; Song, H.; Gan, Q.; Bartoli, F.J.; Kafafi, Z.H. Super absorption of ultra-thin organic photovoltaic films. Opt. Commun. 2014, 314, 48–56. [Google Scholar] [CrossRef]
- Hiramoto, M.; Fujiwara, H.; Yokoyama, M. Three-layered organic solar cell with a photoactive interlayer of codeposited pigments. Appl. Phys. Lett. 1991, 58, 1062–1064. [Google Scholar] [CrossRef]
- Takahashi, K.; Kuraya, N.; Yamaguchi, T.; Komura, T.; Murata, K. Three-layer organic solar cell with high-power conversion efficiency of 3.5%. Sol. Energy Mater. Sol. Cells 2000, 61, 403–416. [Google Scholar] [CrossRef]
- Xu, T.; Qiao, Q. Organic Photovoltaics: Basic Concepts and Device Physics. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2022–2031. ISBN 978-90-481-9751-4. [Google Scholar]
- Zhang, F.; Xu, X.; Tang, W.-H.; Zhang, J.; Zhuo, Z.; Wang, J.; Wang, J.; Xu, Z.; Wang, Y. Recent development of the inverted configuration organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1785–1799. [Google Scholar] [CrossRef]
- Nakano, K.; Tajima, K. Organic Planar Heterojunctions: From Models for Interfaces in Bulk Heterojunctions to High-Performance Solar Cells. Adv. Mater. 2017, 29, 1603269. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Tang, C.W. Hole-transport limited S-shaped I-V curves in planar heterojunction organic photovoltaic cells. Appl. Phys. Lett. 2011, 99, 213506. [Google Scholar] [CrossRef]
- Menke, S.M.; Holmes, R.J. Exciton diffusion in organic photovoltaic cells. Energy Environ. Sci. 2014, 7, 499–512. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Janssen, R.A.J.; Nelson, J. Factors Limiting Device Efficiency in Organic Photovoltaics. Adv. Mater. 2012, 25, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.M. Comparison of Organic Solar Cells and Inorganic Solar Cells. Int. J. Renew. Sustain. Energy 2014, 3, 53. [Google Scholar] [CrossRef]
- Righini, G.C.; Enrichi, F. Solar Cells’ Evolution and Perspectives: A Short Review. In Solar Cells and Light Management; Enrichi, F., Righini, G.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–32. ISBN 978-0-08-102762-2. [Google Scholar]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Geisz, J.F.; Steiner, M.A.; Jain, N.; Schulte, K.L.; France, R.M.; McMahon, W.; Perl, E.E.; Friedman, D.J. Building a Six-Junction Inverted Metamorphic Concentrator Solar Cell. IEEE J. Photovolt. 2018, 8, 626–632. [Google Scholar] [CrossRef]
- Mateker, W.R.; McGehee, M.D. Progress in Understanding Degradation Mechanisms and Improving Stability in Organic Photovoltaics. Adv. Mater. 2017, 29, 1603940. [Google Scholar] [CrossRef]
- Channa, I.; Distler, A.; Zaiser, M.; Brabec, C.J.; Egelhaaf, H.-J. Thin Film Encapsulation of Organic Solar Cells by Direct Deposition of Polysilazanes from Solution. Adv. Energy Mater. 2019. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, C.; Zhan, X. Morphology Control in Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1703147. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Ratier, B.; Nunzi, J.-M.; Aldissi, M.; Kraft, T.M.; Buncel, E. Organic solar cell materials and active layer designs-improvements with carbon nanotubes: A review. Polym. Int. 2012, 61, 342–354. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Liu, X.; Gu, Y. Research progress on polymer heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2012, 98, 129–145. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, C.; Dong, S.; Jiang, X.; Wu, S.; Wu, H.; Yip, H.-L.; Huang, F.; Cao, Y. n-Type Water/Alcohol-Soluble Naphthalene Diimide-Based Conjugated Polymers for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.P.; Yiu, A.T.; Beaujuge, P.M.; Woo, C.H.; Holcombe, T.W.; Millstone, J.E.; Douglas, J.D.; Chen, M.; Frechet, J. Efficient Small Molecule Bulk Heterojunction Solar Cells with High Fill Factors via Pyrene-Directed Molecular Self-Assembly. Adv. Mater. 2011, 23, 5359–5363. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dye. Pigment. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2016, 22, 21. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Jiang, H.; Wang, X.; Yan, L.; Zeng, W.; Wu, X.-G.; Zhuang, H.; Zhu, W.; Yang, R. Steady Enhancement in Photovoltaic Properties of Fluorine Functionalized Quinoxaline-Based Narrow Bandgap Polymer. Molecules 2018, 24, 54. [Google Scholar] [CrossRef] [Green Version]
- Aivali, S.; Tsimpouki, L.; Anastasopoulos, C.; Kallitsis, J. Synthesis and Optoelectronic Characterization of Perylene Diimide-Quinoline Based Small Molecules. Molecules 2019, 24, 4406. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular Design of Benzodithiophene-Based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef] [PubMed]
- Gedefaw, D.A.; Prosa, M.; Bolognesi, M.; Seri, M.; Andersson, M.R. Recent Development of Quinoxaline Based Polymers/Small Molecules for Organic Photovoltaics. Adv. Energy Mater. 2017, 7, 1700575. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical Review of the Molecular Design Progress in Non-Fullerene Electron Acceptors Towards Commercial-ly Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef] [PubMed]
- Camic, B.; Jeong, H.; Aslan, M.; Kosemen, A.; Kim, S.; Choi, H.; Basarir, F.; Lee, B.R. Preparation of Transparent Conductive Electrode via Layer-By-Layer Deposition of Silver Nanowires and Its Application in Organic Photovoltaic Device. Nanomaterials 2020, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Oprea, C.; Gîrțu, M. Structure and Electronic Properties of TiO2 Nanoclusters and Dye–Nanocluster Systems Appropriate to Model Hybrid Photovoltaic or Photocatalytic Applications. Nanomaterials 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, I.; Bazaka, K.; Keidar, M.; Xu, S.; Fang, J. Hierarchical Multicomponent Inorganic Metamaterials: Intrinsically Driven Self-Assembly at the Nanoscale. Adv. Mater. 2018, 30, 1702226. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Liang, N.; Yao, H.; Wei, Z.; He, C.; Yuan, X.; Hou, J. Effects of Energy-Level Offset between a Donor and Acceptor on the Photovoltaic Performance of Non-Fullerene Organic Solar Cells. J. Mater. Chem. A 2019, 7, 18889–18897. [Google Scholar] [CrossRef]
- Choy, W.C. Organic Solar Cells: Materials and Device Physics; Springer: Dordrecht, The Netherlands, 2012; ISBN 1-4471-4823-1. [Google Scholar]
- Amusan, O.O.; Louis, H.; Zafar, S.-; Hamzat, A.T.; Peter, D.M. Different Interface Engineering in Organic Solar Cells: A Review. Chem. Methodol. 2019, 3, 425–441. [Google Scholar] [CrossRef]
- Ma, H.; Yip, H.-L.; Huang, F.; Jen, A. Interface Engineering for Organic Electronics. Adv. Funct. Mater. 2010, 20, 1371–1388. [Google Scholar] [CrossRef]
- Huang, Y.; Kramer, E.J.; Heeger, A.J.; Bazan, G.C. Bulk Heterojunction Solar Cells: Morphology and Performance Relationships. Chem. Rev. 2014, 114, 7006–7043. [Google Scholar] [CrossRef]
- Treat, N.D.; Chabinyc, M.L. Phase Separation in Bulk Heterojunctions of Semiconducting Polymers and Fullerenes for Photovoltaics. Annu. Rev. Phys. Chem. 2014, 65, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Hoefler, S.F.; Haberfehlner, G.; Rath, T.; Keilbach, A.; Hobisch, M.A.; Dixon, A.; Pavlica, E.; Bratina, G.; Kothleitner, G.; Hofer, F.; et al. Elucidation of Donor:Acceptor Phase Separation in Nonfullerene Organic Solar Cells and Its Implications on Device Performance and Charge Carrier Mobility. ACS Appl. Energy Mater. 2019, 2, 7535–7545. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, N.; Zhou, W.; Yu, Z.; Liu, F.; Chen, Y. Miscibility Tuning for Optimizing Phase Separation and Vertical Distribution toward Highly Efficient Organic Solar Cells. Adv. Sci. 2019, 6, 1900565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.-L.; Wu, B.; Zheng, F.; Wang, H.-B.; Wang, Y.; Bian, F.; Hao, X.-T.; Zhu, F. Homogeneous phase separation in polymer: Fullerene bulk heterojunction organic solar cells. Org. Electron. 2015, 25, 266–274. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia-Belmonte, G. Recent Advances to Understand Morphology Stability of Organic Photovoltaics. Nano-Micro Lett. 2016, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, C.J.; Palumbiny, C.M.; Niedermeier, M.A.; Jendrzejewski, C.; Santoro, G.; Roth, S.V.; Müller-Buschbaum, P. A Direct Evidence of Morphological Degradation on a Nanometer Scale in Polymer Solar Cells. Adv. Mater. 2013, 25, 6760–6764. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Huang, Y.-C.; Liu, W.-S.; Su, Y.-A.; Tsao, C.-S.; Rwei, S.-P.; Wang, L. Insights into the Morphological Instability of Bulk Heterojunction PTB7-Th/PCBM Solar Cells upon High-Temperature Aging. ACS Appl. Mater. Interfaces 2017, 9, 14808–14816. [Google Scholar] [CrossRef]
- Sherafatipour, G.; Benduhn, J.; Patil, B.R.; Ahmadpour, M.; Spoltore, D.; Rubahn, H.-G.; Vandewal, K.; Madsen, M. Degradation pathways in standard and inverted DBP-C70 based organic solar cells. Sci. Rep. 2019, 9, 4024. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Xin, J.; Ma, W. Hierarchical Morphology Stability under Multiple Stresses in Organic Solar Cells. ACS Energy Lett. 2019, 4, 447–455. [Google Scholar] [CrossRef]
- Fabiano, S.; Pignataro, B. Engineering 3D ordered molecular thin films by nanoscale control. Phys. Chem. Chem. Phys. 2010, 12, 14848. [Google Scholar] [CrossRef]
- Fabiano, S.; Chen, Z.; Vahedi, S.; Facchetti, A.; Pignataro, B.; Loi, M.A. Role of Photoactive Layer Morphology in High Fill Factor All-Polymer Bulk Heterojunction Solar Cells. J. Mater. Chem. 2011, 21, 5891–5896. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, S.; Sartorio, C.; Giannazzo, F.; Scandurra, A.; Pignataro, B.G. Self-organization and nanostructural control in thin film heterojunctions. Nanoscale 2014, 6, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, C.; Scaramuzza, S.; Cataldo, S.; Vetri, V.; Scopelliti, M.; Leone, M.; Amendola, V.; Pignataro, B. Donor–Acceptor Interfaces by Engineered Nanoparticles Assemblies for Enhanced Efficiency in Plastic Planar Heterojunction Solar Cells. J. Phys. Chem. C 2016, 120, 26588–26599. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent Advances in Morphology Optimization for Organic Photovoltaics. Adv. Mater. 2018, 30, 1800453. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Zheng, D.; Yu, J. Effects of Different Polar Solvents for Solvent Vapor Annealing Treatment on the Performance of Polymer Solar Cells. Org. Electron. 2014, 15, 2647–2653. [Google Scholar] [CrossRef]
- Hood, S.; Kassal, I. Entropy and Disorder Enable Charge Separation in Organic Solar Cells. J. Phys. Chem. Lett. 2016, 7, 4495–4500. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, N.; Salleo, A.; McCulloch, I.; Baran, D. The role of the third component in ternary organic solar cells. Nat. Rev. Mater. 2019, 4, 229–242. [Google Scholar] [CrossRef]
- Sartorio, C.; Giuliano, G.; Scopelliti, M.; Vetri, V.; Leone, M.; Pignataro, B. Synergies and compromises between charge and energy transfers in three-component organic solar cells. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2002; Volume 1, ISBN 1-4020-1110-5. [Google Scholar]
- Lombeck, F.; Sepe, A.; Thomann, R.; Friend, R.H.; Sommer, M. Compatibilization of All-Conjugated Polymer Blends for Organic Photovoltaics. ACS Nano 2016, 10, 8087–8096. [Google Scholar] [CrossRef]
- Xu, X.; Fukuda, K.; Karki, A.; Park, S.; Kimura, H.; Jinno, H.; Watanabe, N.; Yamamoto, S.; Shimomura, S.; Kitazawa, D.; et al. Thermally Stable, Highly Efficient, Utraflexible Organic Photovoltaics. Proc. Natl. Acad. Sci. USA 2018, 115, 4589–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Shrotriya, V.; Yao, Y.; Huang, J.; Yang, Y. Manipulating Regioregular Poly(3-Hexylthiophene): [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Blends—Route towards High Efficiency Polymer Solar Cells. J. Mater. Chem. 2007, 17, 3126. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the Morphology and Performance of Bulk Het-erojunctions in Solar Cells. Lessons Learned from the Benchmark Poly(3-hexylthiophene):[6,6]-Phenyl-C61-butyric Acid Methyl Ester System. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Fullerene-Bisadduct Acceptors for Polymer Solar Cells. Chem. Asian J. 2013, 8, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Karak, S.; Page, Z.; Li, S.; Tinkham, J.S.; Lahti, P.M.; Duzhko, V.; Emrick, T. Amino-fulleropyrrolidines as electrotropic additives to enhance organic photovoltaics. Sustain. Energy Fuels 2018, 2, 2143–2147. [Google Scholar] [CrossRef]
- Moon, J.S.; Takacs, C.J.; Cho, S.; Coffin, R.C.; Kim, H.; Bazan, G.C.; Heeger, A.J. Effect of Processing Additive on the Nanomorphology of a Bulk Heterojunction Material. Nano Lett. 2010, 10, 4005–4008. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC press: Boca Raton, FL, USA, 2002; ISBN 1-4200-4931-3. [Google Scholar]
- Vongsaysy, U.; Pavageau, B.; Wantz, G.; Bassani, D.M.; Servant, L.; Aziz, H. Guiding the Selection of Processing Additives for Increasing the Efficiency of Bulk Heterojunction Polymeric Solar Cells. Adv. Energy Mater. 2014, 4, 1300752. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Z.; Ma, W.; Xie, Z.; Liu, J.; Yu, X.; Han, Y. Efficient Nonhalogenated Solvent-Processed Ternary All-Polymer Solar Cells with a Favorable Morphology Enabled by Two Well-Compatible Donors. ACS Appl. Mater. Interfaces 2019, 11, 32200–32208. [Google Scholar] [CrossRef]
- Zhang, C.; Heumueller, T.; Leon, S.; Gruber, W.; Burlafinger, K.; Tang, X.; Perea, J.D.; Wabra, I.; Hirsch, A.; Unruh, T.; et al. A Top-Down Strategy Identifying Molecular Phase Stabilizers to Overcome Microstructure Instabilities in Organic Solar Cells. Energy Environ. Sci. 2019, 12, 1078–1087. [Google Scholar] [CrossRef]
- Sivula, K.; Ball, Z.T.; Watanabe, N.; Fréchet, J.M. Amphiphilic Diblock Copolymer Compatibilizers and Their Effect on the Morphology and Performance of Polythiophene: Fullerene Solar Cells. Adv. Mater. 2006, 18, 206–210. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for Morphology Control in High-Efficiency Organic Solar Cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, L.; Chen, Y. Nanostructuring Compatibilizers of Block Copolymers for Organic Photovoltaics. Polym. Int. 2014, 63, 593–606. [Google Scholar] [CrossRef]
- Sun, L.; Xu, X.; Song, S.; Zhang, Y.; Miao, C.; Liu, X.; Xing, G.; Zhang, S. Medium-Bandgap Conjugated Polymer Donors for Organic Photovoltaics. Macromol. Rapid Commun. 2019, 40, e1900074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Yang, K.; Chen, P.; Guo, X. Additive-Free Non-Fullerene Organic Solar Cells. ChemELectroChem 2019, 6, 5547–5562. [Google Scholar] [CrossRef]
- Liao, H.-C.; Chen, P.-H.; Chang, R.P.H.; Su, W.-F. Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells. Polymers 2014, 6, 2784–2802. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Kelly, M.A.; You, W.; Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photonics 2015, 9, 491–500. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef]
- Liao, H.-C.; Tsao, C.-S.; Lin, T.-H.; Jao, M.-H.; Chuang, C.-M.; Chang, S.-Y.; Huang, Y.-C.; Shao, Y.-T.; Chen, C.-Y.; Su, C.-J.; et al. Nanoparticle-Tuned Self-Organization of a Bulk Heterojunction Hybrid Solar Cell with Enhanced Performance. ACS Nano 2012, 6, 1657–1666. [Google Scholar] [CrossRef]
- Majkić, A.; Gadermaier, C.; Ćelić, N.; Topolovsek, P.; Bratina, G.; Mihailovic, D. Mo6S9−xIx nanowires as additives for enhanced organic solar cell performance. Sol. Energy Mater. Sol. Cells 2014, 127, 63–66. [Google Scholar] [CrossRef]
- Cataldo, S.; Fabiano, S.; Ferrante, F.; Previti, F.; Patanè, S.; Pignataro, B. Organoboron Polymers for Photovoltaic Bulk Heterojunctions. Macromol. Rapid Commun. 2010, 31, 1281–1286. [Google Scholar] [CrossRef]
- Cataldo, S.; Pignataro, B.G. Polymeric Thin Films for Organic Electronics: Properties and Adaptive Structures. Materials 2013, 6, 1159–1190. [Google Scholar] [CrossRef] [Green Version]
- Marrocchi, A.; Lanari, D.; Facchetti, A.; Vaccaro, L. Poly(3-hexylthiophene): Synthetic methodologies and properties in bulk heterojunction solar cells. Energy Environ. Sci. 2012, 5, 8457. [Google Scholar] [CrossRef]
- Marks, R.N.; Halls, J.J.M.; Bradley, D.D.C.; Friend, R.H.; Holmes, A.B. The photovoltaic response in poly(p-phenylene vinylene) thin-film devices. J. Phys. Condens. Matter 1994, 6, 1379–1394. [Google Scholar] [CrossRef]
- Mulherin, R.C.; Jung, S.; Huettner, S.; Johnson, K.; Kohn, P.; Sommer, M.; Allard, S.; Scherf, U.; Greenham, N.C. Ternary Photovoltaic Blends Incorporating an All-Conjugated Donor–Acceptor Diblock Copolymer. Nano Lett. 2011, 11, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Kim, H.; Ma, B.; Seo, H.; Park, J.-W. Photovoltaic Efficiency Enhancement by the Generation of an Embedded Silica-Like Passivation Layer along the P3HT/PCBM Interface Using an Asymmetric Block-Copolymer Additive. Adv. Mater. 2012, 24, 6311–6317. [Google Scholar] [CrossRef]
- Lobez, J.M.; Andrew, T.L.; Bulović, V.; Swager, T.M. Improving the Performance of P3HT–Fullerene Solar Cells with Side-Chain-Functionalized Poly(thiophene) Additives: A New Paradigm for Polymer Design. ACS Nano 2012, 6, 3044–3056. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Huang, P.-T.; Lin, K.-C.; Huang, Y.-J.; Chen, C.-T. Stabilization of Poly(3-Hexylthiophene)/PCBM Morphology by Hydroxyl Group End-Functionalized P3HT and its Application to Polymer Solar Cells. Org. Electron. 2012, 13, 283–289. [Google Scholar] [CrossRef]
- Renaud, C.; Mougnier, S.-J.; Pavlopoulou, E.; Brochon, C.; Fleury, G.; Deribew, D.; Portale, G.; Cloutet, E.; Chambon, S.; Vignau, L.; et al. Block Copolymer as a Nanostructuring Agent for High-Efficiency and Annealing-Free Bulk Heterojunction Organic Solar Cells. Adv. Mater. 2012, 24, 2196–2201. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.-H.; Ryu, J.-H.; Kim, Y.; Kang, H.; Lee, W.B.; Kim, T.-S.; Kim, B.J. Architectural Engineer-ing of Rod–Coil Compatibilizers for Producing Mechanically and Thermally Stable Polymer Solar Cells. ACS Nano 2014, 8, 10461–10470. [Google Scholar] [CrossRef]
- Lin, J.-F.; Yen, W.-C.; Chang, C.-Y.; Chen, Y.-F.; Su, W.-F. Enhancing organic–inorganic hybrid solar cell efficiency using rod–coil diblock polymer additive. J. Mater. Chem. A 2013, 1, 665–670. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Yin, W.; Yu, X.; Shao, M.; Xiao, K.; Hong, K.; Pickel, D.L.; Kochemba, W.M.; Ii, S.M.K.; et al. Correlation of polymeric compatibilizer structure to its impact on the morphology and function of P3HT:PCBM bulk heterojunctions. J. Mater. Chem. A 2013, 1, 5309. [Google Scholar] [CrossRef]
- Fujita, H.; Michinobu, T.; Fukuta, S.; Koganezawa, T.; Higashihara, T. Sequentially Different AB Diblock and ABA Triblock Copolymers as P3HT:PCBM Interfacial Compatibilizers for Bulk-Heterojunction Photovoltaics. ACS Appl. Mater. Interfaces 2016, 8, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Kim, H.; Jang, Y.J.; Park, S.; Ryu, D.Y.; Kim, K.; Tang, P.; Qiu, F.; Kim, D.H.; Peng, J. Toward high efficiency organic photovoltaic devices with enhanced thermal stability utilizing P3HT-b-P3PHT block copolymer additives. J. Mater. Chem. A 2016, 4, 18432–18443. [Google Scholar] [CrossRef]
- Sun, Y.; Pitliya, P.; Liu, C.; Gong, X.; Raghavan, D.; Karim, A. Block copolymer compatibilized polymer: Fullerene blend morphology and properties. Polymers 2017, 113, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Xiao, K.; Keum, J.K.; Yu, X.; Hong, K.; Browning, J.; Ivanov, I.N.; Chen, J.; Alonzo, J.; Li, D.; et al. PS-b-P3HT Copolymers as P3HT/PCBM Interfacial Compatibilizers for High Efficiency Photovoltaics. Adv. Mater. 2011, 23, 5529–5535. [Google Scholar] [CrossRef]
- Mohammadi-Arbati, E.; Agbolaghi, S. Efficiency above 6% in poly(3-hexylthiophene): Phenyl-C-butyric acid methyl ester photovoltaics via simultaneous addition of poly(3-hexylthiophene) based grafted graphene nanosheets and hydrophobic block copolymers. Polym. Int. 2019, 68, 1292–1302. [Google Scholar] [CrossRef]
- Xu, B.; Saianand, G.; Roy, V.A.L.; Qiao, Q.; Reza, K.M.; Kang, X.-W. Employing PCBTDPP as an Efficient Donor Polymer for High Performance Ternary Polymer Solar Cells. Polymers 2019, 11, 1423. [Google Scholar] [CrossRef] [Green Version]
- Lei, T.; Peng, R.; Fan, X.; Wei, Q.; Liu, Z.; Guan, Q.; Song, W.; Hong, L.; Huang, J.; Yang, R.; et al. Highly Efficient Non-Fullerene Organic Solar Cells Using 4,8-Bis((2-ethylhexyl)oxy)benzo[1,2-b:4,5-b′]dithiophene-Based Polymers as Additives. Macromolecules 2018, 51, 4032–4039. [Google Scholar] [CrossRef]
- Zhang, K.-N.; Yang, X.-Y.; Niu, M.-S.; Wen, Z.-C.; Chen, Z.-H.; Feng, L.; Feng, X.-J.; Hao, X.-T. Modulating the Morphology and Molecular Arrangement via the Well-Compatible Polymer Donor in Multiple Working Mechanisms Interwined Ternary Organic Solar Cells. Org. Electron. 2019, 66, 13–23. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Zhou, W.; Yu, Z.; Chen, L.; Liu, F.; Chen, Y. Vertical Distribution to Optimize Active Layer Morphology for Efficient All-Polymer Solar Cells by J71 as a Compatibilizer. Macromolecules 2019, 52, 4359–4369. [Google Scholar] [CrossRef]
- Rattanathamwat, N.; Wootthikanokkhan, J.; Nimitsiriwat, N.; Thanachayanont, C.; Asawapirom, U.; Keawprajak, A. Poly(3-hexyl thiophene)-b-Fullerene Functionalized Polystyrene Copolymers (P3HT-b-PSFu) as Compatibilizer in P3HT/Phenyl-C61-butyric Acid Methyl Ester (PCBM) Solar Cells. Int. J. Polym. Mater. 2014, 63, 476–485. [Google Scholar] [CrossRef]
- Lee, J.U.; Jung, J.-W.; Emrick, T.; Russell, T.P.; Jo, W.H. Morphology control of a polythiophene–fullerene bulk heterojunction for enhancement of the high-temperature stability of solar cell performance by a new donor–acceptor diblock copolymer. Nanotechnology 2010, 21, 105201. [Google Scholar] [CrossRef] [PubMed]
- Bicciocchi, E.; Haeussler, M.; Rizzardo, E.; Scully, A.D.; Ghiggino, K.P. Donor–Acceptor Rod–Coil Block Copolymers Comprising Poly[2,7-(9,9-Dihexylfluorene)-alt-Bithiophene] and Fullerene as Compatibilizers for Organic Photovoltaic Devices. J. Polym. Sci. Pol. Chem. 2015, 53, 888–903. [Google Scholar] [CrossRef]
- Kakogianni, S.; Andreopoulou, A.K.; Kallitsis, J.K. Synthesis of Polythiophene–Fullerene Hybrid Additives as Potential Compatibilizers of BHJ Active Layers. Polymers 2016, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- Sartorio, C.; Campisciano, V.; Chiappara, C.; Cataldo, S.; Scopelliti, M.; Gruttadauria, M.; Giacalone, F.; Pignataro, B.G. Enhanced power-conversion efficiency in organic solar cells incorporating copolymeric phase-separation modulators. J. Mater. Chem. A 2018, 6, 3884–3894. [Google Scholar] [CrossRef]
- Chen, M.-C.; Liaw, D.-J.; Chen, W.-H.; Huang, Y.-C.; Sharma, J.; Tai, Y. Improving the Efficiency of an Organic Solar Cell by a Polymer Additive to Optimize the Charge Carriers Mobility. Appl. Phys. Lett. 2011, 99, 223305. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Chen, M.-C.; Liaw, D.-J.; Wu, H.-Y.; Huang, Y.-C.; Tai, Y. A Bifunctional Copolymer Additive to Utilize Photoenergy Transfer and To Improve Hole Mobility for Organic Ternary Bulk-Heterojunction Solar Cell. ACS Appl. Mater. Interfaces 2014, 6, 12119–12125. [Google Scholar] [CrossRef]
- Mok, J.W.; Kipp, D.; Hasbun, L.R.; Dolocan, A.; Strzalka, J.; Ganesan, V.; Verduzco, R. Parallel Bulk Heterojunction Photovoltaics Based on All-Conjugated Block Copolymer Additives. J. Mater. Chem. A 2016, 4, 14804–14813. [Google Scholar] [CrossRef]
- Lee, W.; Jeong, S.; Lee, C.; Han, G.; Cho, C.; Lee, J.-Y.; Kim, B.J. Self-Organization of Polymer Additive, Poly(2-vinylpyridine) via One-Step Solution Processing to Enhance the Efficiency and Stability of Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1602812. [Google Scholar] [CrossRef]
- Chen, W.-J.; Cheng, Y.-C.; Kuo, D.-W.; Chen, C.-T.; Liu, B.-T.; Jeng, R.-J.; Lee, R.-H. A Star-Shaped Conjugated Molecule Featuring a Triazole Core and Diketopyrrolopyrrole Branches is an Efficient Electron-Selective Interlayer for Inverted Polymer Solar Cells. RSC Adv. 2018, 8, 31478–31489. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Li, X.; Liang, Z.; Huang, G.; Chen, W.; Zheng, N.; Yang, R. Employing Structurally Similar Acceptors as Crystalline Modulators to Construct High Efficiency Ternary Organic Solar Cells. J. Mater. Chem. A 2019, 7, 7760–7765. [Google Scholar] [CrossRef]
- Peng, B.; Guo, X.; Zou, Y.; Pan, C.; Li, Y. Performance improvement of annealing-free P3HT: PCBM-based polymer solar cells via 3-methylthiophene additive. J. Phys. D Appl. Phys. 2011, 44, 365101. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Unni, G.E.; Jeong, H.-M.; Kim, J.-S.; Kim, S.-W.; Kwon, J.-B.; Bae, J.-H.; Kang, S.-W. Pyridine-based additive optimized P3HT:PC61BM nanomorphology for improved performance and stability in polymer solar cells. Appl. Surf. Sci. 2019, 484, 825–834. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Inoue, K.; Harano, K.; Tanaka, H.; Nakamura, E. Enhancement in the Efficiency of an Organic–Inorganic Hybrid Solar Cell with a Doped P3HT Hole-Transporting Layer on a Void-Free Perovskite Active Layer. J. Mater. Chem. A 2014, 2, 13827–13830. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Saianand, G.; Gopalan, A.-I.; Muthuchamy, N.; Lee, K.-P.; Lee, J.-S.; Jiang, Y.; Lee, S.-W.; Kim, S.-W.; Kim, J.-S.; et al. Functional solid additive modified PEDOT:PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Saianand, G.; Dubey, A.; Saianand, G.; Venkatesan, S.; Ruban, S.; Reza, K.M.; Choi, J.; Lakhi, K.; Xu, B.; Qiao, Q.; et al. Additive assisted morphological optimization of photoactive layer in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 182, 246–254. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, Y.; Shi, G.; Ling, X.; Ying, L.; Huang, F.; Lee, T.H.; Woo, H.Y.; Kim, J.Y.; Cao, Y.; et al. Engineering the Morphology via Processing Additives in Multiple All-Polymer Solar Cells for Improved Performance. J. Mater. Chem. A 2018, 6, 10421–10432. [Google Scholar] [CrossRef]
- Xie, S.; Wang, J.; Wang, R.; Zhang, N.; Zhou, H.; Zhang, Y.; Zhou, D. Effects of processing additives in non-fullerene organic bulk heterojunction solar cells with efficiency >11%. Chin. Chem. Lett. 2019, 30, 217–221. [Google Scholar] [CrossRef]
- Zheng, Y.; Goh, T.; Fan, P.; Shi, W.; Yu, J.; Taylor, A.D. Toward Efficient Thick Active PTB7 Photovoltaic Layers Using Diphenyl Ether as a Solvent Additive. ACS Appl. Mater. Interfaces 2016, 8, 15724–15731. [Google Scholar] [CrossRef]
- Laventure, A.; Harding, C.R.; Cieplechowicz, E.; Li, Z.; Wang, J.; Zou, Y.; Welch, G.C. Screening Quinoxaline-Type Donor Polymers for Roll-to-Roll Processing Compatible Organic Photovoltaics. ACS Appl. Polym. Mater. 2019, 1, 2168–2176. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, M.; Park, C.W.; Kim, H.U.; Lee, H.-K.; Park, T. Morphological Control of Donor/Acceptor Interfaces in All-Polymer Solar Cells Using a Pentafluorobenzene-Based Additive. Chem. Mater. 2017, 29, 6793–6798. [Google Scholar] [CrossRef]
- Liu, X.; Huettner, S.; Rong, Z.; Sommer, M.; Friend, R.H. Solvent Additive Control of Morphology and Crystallization in Semiconducting Polymer Blends. Adv. Mater. 2011, 24, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, F.; Cao, Y.; Chen, J. Low Boiling Point Solvent Additives Enable Vacuum Drying-Free Processed 230 nm Thick PTB7-Th:PC71BM Active Layers with More than 10% Power Conversion Efficiency. J. Mater. Chem. A 2019, 7, 1861–1869. [Google Scholar] [CrossRef]
- Min, J.; Kwon, O.K.; Cui, C.; Park, J.-H.; Wu, Y.; Park, S.Y.; Li, Y.; Brabec, C.J. High performance all-small-molecule solar cells: Engineering the nanomorphology via processing additives. J. Mater. Chem. A 2016, 4, 14234–14240. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yeo, J.-S.; Jeong, H.-G.; Yun, J.-M.; Kim, Y.-A.; Kim, D.-Y. A Thienylenevinylene-Phthalimide Co-polymer Based Polymer Solar Cell with High Open Circuit Voltage: Effect of Additive Concentration on the Open Circuit Voltage. Sol. Energy Mater. Sol. Cells 2014, 125, 253–260. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Z.; Duan, C.; Wang, Z.; Yin, Q.; Huang, F.; Cao, Y. Polythiophene derivatives compatible with both fullerene and non-fullerene acceptors for polymer solar cells. J. Mater. Chem. C 2019, 7, 314–323. [Google Scholar] [CrossRef]
- Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; El Labban, A.; Tassone, C.J.; Toney, M.F.; Frechet, J.; Beaujuge, P.M.; McGehee, M.D. Controlling Solution-Phase Polymer Aggregation with Molecular Weight and Solvent Additives to Optimize Polymer-Fullerene Bulk Heterojunction Solar Cells. Adv. Energy Mater. 2014, 4, 1301733. [Google Scholar] [CrossRef]
- Chueh, C.-C.; Yao, K.; Yip, H.-L.; Chang, C.-Y.; Xu, Y.-X.; Chen, K.-S.; Li, C.-Z.; Liu, P.; Huang, F.; Chen, Y.; et al. Non-halogenated solvents for environmentally friendly processing of high-performance bulk-heterojunction polymer solar cells. Energy Environ. Sci. 2013, 6, 3241. [Google Scholar] [CrossRef]
- Aïch, B.R.; Beaupré, S.; Leclerc, M.; Tao, Y. Highly efficient thieno[3,4-c]pyrrole-4,6-dione-based solar cells processed from non-chlorinated solvent. Org. Electron. 2014, 15, 543–548. [Google Scholar] [CrossRef]
- Jhuo, H.-J.; Liao, S.-H.; Li, Y.-L.; Yeh, P.-N.; Chen, S.-A.; Wu, W.-R.; Su, C.-J.; Lee, J.-J.; Yamada, N.L.; Jeng, U.-S. The Novel Additive 1-Naphthalenethiol Opens a New Processing Route to Efficiency-Enhanced Polymer Solar Cells. Adv. Funct. Mater. 2016, 26, 3094–3104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Li, L.; An, Q.; Wang, J.; Zhang, J. The Underlying Reason of DIO Additive on the Improvement Polymer Solar Cells Performance. Appl. Surf. Sci. 2014, 305, 221–226. [Google Scholar] [CrossRef]
- Yu, R.; Yao, H.; Hong, L.; Qin, Y.; Zhu, J.; Cui, Y.; Li, S.; Hou, J. Design and Application of Volatilizable Solid Additives in Non-Fullerene Organic Solar Cells. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frankenstein, H.; Leng, C.Z.; Losego, M.D.; Frey, G.L. Atomic Layer Deposition of ZnO Electron Transporting Layers Directly onto the Active Layer of Organic Solar Cells. Org. Electron. 2019, 64, 37–46. [Google Scholar] [CrossRef]
- Ou, R.-X.; Lin, C.-H.; Guo, T.-F.; Wen, T.-C. Improvement in inverted polymer solar cells via 1-benzoyl-2-thiourea as surface modifier on sol-gel ZnO. J. Taiwan Inst. Chem. Eng. 2019, 96, 131–136. [Google Scholar] [CrossRef]
- Fanady, B.; Song, W.; Peng, R.; Wu, T.; Ge, Z. Efficiency Enhancement of Organic Solar Cells Enabled by Interface Engineering of Dol-Gel Zinc Oxide with an Oxadiazole-Based Material. Org. Electron. 2020, 76, 105483. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Fan, P.; Yang, X.; Yu, J. Enhanced Power Conversion Efficiency of P3HT: PC 71 BM Bulk Heterojunction Polymer Solar Cells by Doping a High-Mobility Small Organic Molecule. Int. J. Photoenergy 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gundlach, D.J.; Lin, Y.Y.; Jackson, T.N.; Nelson, S.F.; Schlom, D.G. Pentacene Organic Thin-Film Transistors-Molecular Ordering and Mobility. IEEE Electron Device Lett. 1997, 18, 87–89. [Google Scholar] [CrossRef]
- Maliakal, A.; Raghavachari, K.; Katz, H.; Chandross, E.; Siegrist, T. Photochemical Stability of Pentacene and a Substituted Pentacene in Solution and in Thin Films. Chem. Mater. 2004, 16, 4980–4986. [Google Scholar] [CrossRef]
- Kim, J.B.; Allen, K.; Oh, S.J.; Lee, S.; Toney, M.F.; Kim, Y.S.; Kagan, C.R.; Nuckolls, C.; Loo, Y.-L. Small-Molecule Thiophene-C60Dyads As Compatibilizers in Inverted Polymer Solar Cells. Chem. Mater. 2010, 22, 5762–5773. [Google Scholar] [CrossRef]
- Mei, Q.; Li, C.; Gong, X.; Lu, H.; Jin, E.; Du, C.; Lu, Z.; Jiang, L.; Meng, X.; Wang, C.; et al. Enhancing the Performance of Polymer Photovoltaic Cells by Using an Alcohol Soluble Fullerene Derivative as the Interfacial Layer. ACS Appl. Mater. Interfaces 2013, 5, 8076–8080. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Liu, W.-S.; Hsiow, C.-Y.; Rwei, S.-P.; Chiu, W.-Y.; Wang, L. Terthiophene–C60 Dyads as Donor/Acceptor Compatibilizers for Developing Highly Stable P3HT/PCBM Bulk Heterojunction Solar Cells. J. Mater. Chem. A 2015, 3, 14401–14408. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the Bright Future—Bulk Heterojunction Polymer Solar Cells with Power Conversion Efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.-R.; Wakim, S.; Zhou, J.; Leclerc, M.; Li, Z.; Ding, J.; et al. Bulk Heterojunction Solar Cells Using Thieno[3,4-c]pyrrole-4,6-dione and Dithieno[3,2-b:2′,3′-d]silole Copolymer with a Power Conversion Efficiency of 7.3%. J. Am. Chem. Soc. 2011, 133, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.J.; Szarko, J.M.; Xu, T.; Yu, L.; Marks, T.J.; Chen, L.X. Effects of Additives on the Morphology of Solution Phase Aggregates Formed by Active Layer Components of High-Efficiency Organic Solar Cells. J. Am. Chem. Soc. 2011, 133, 20661–20663. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, S.; Imamura, S.; Kannappan, S.; Palanisamy, K.; Shin, P.-K. Characteristics and the Effect of Additives on the Nanomorphology of PTB7/PC71BM Composite Films. Curr. Appl. Phys. 2013, 13, S58–S63. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Lou, S.J.; Smith, J.; Tice, D.B.; Hennek, J.W.; Ortiz, R.P.; Navarrete, J.T.L.; Li, S.; Strzalka, J.; et al. Polymer solar cells with enhanced fill factors. Nat. Photonics 2013, 7, 825–833. [Google Scholar] [CrossRef]
- Guan, Z.; Yu, J.; Huang, J.; Zhang, L. Power efficiency enhancement of solution-processed small-molecule solar cells based on squaraine via thermal annealing and solvent additive methods. Sol. Energy Mater. Sol. Cells 2013, 109, 262–269. [Google Scholar] [CrossRef]
- Wang, N.H.; Morin, P.-O.; Lee, C.-L.; Kyaw, A.K.K.; Leclerc, M.; Heeger, A.J. Effect of processing additive on morphology and charge extraction in bulk-heterojunction solar cells. J. Mater. Chem. A 2014, 2, 15052–15057. [Google Scholar] [CrossRef]
- Collins, B.A.; Li, Z.; Tumbleston, J.R.; Gann, E.; McNeill, C.R.; Ade, H. Organic Solar Cells: Absolute Measurement of Domain Composition and Nanoscale Size Distribution Explains Performance in PTB7:PC71 BM Solar Cells. Adv. Energy Mater. 2013, 3, 65–74. [Google Scholar] [CrossRef]
- Salim, T.; Bräuer, B.; Kukreja, R.; Foo, Y.L.; Bao, Z.; Lam, Y.M.; Wong, L.H. Solvent additives and their effects on blend morphologies of bulk heterojunctions. J. Mater. Chem. 2011, 21, 242–250. [Google Scholar] [CrossRef]
- Rogers, J.T.; Schmidt, K.; Toney, M.F.; Kramer, E.J.; Bazan, G.C. Structural Order in Bulk Heterojunction Films Prepared with Solvent Additives. Adv. Mater. 2011, 23, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Tremolet de Villers, B.J.; O’Hara, K.A.; Ostrowski, D.P.; Biddle, P.H.; Shaheen, S.E.; Chabinyc, M.L.; Olson, D.C.; Kopidakis, N. Removal of Residual Diiodooctane Improves Photostability of High-Performance Organic Solar Cell Polymers. Chem. Mater. 2016, 28, 876–884. [Google Scholar] [CrossRef]












| CBs | Definition (according to the Reference) | Major Role(s) | Compatibilized Blend |
|---|---|---|---|
| 5 | modulator [151] | morphology modulator | P3HT:PCBM |
| P3HT-b-PTCNE | compatibilizer [138] | morphology modulator, interface stabilizer | P3HT:PCBM |
| F6T2-b-PS(PCBM) | compatibilizer [149] | increasing operational stability | F6T2:PCBM |
| HOC-P3HT-COH | compatibilizer [133] | morphology stabilizer, thermal stress stabilizer | P3HT:PCBM |
| J71 | compatibilizer [146] | morphology modulator, interface stabilizer, absorption range extender, energy levels matcher | PBDB-T:PNDI-2T-TR |
| P2VP | additive [155] | morphology modulator, device stabilizer | PTB7:PCBM |
| P3HT-(P3HT-5F)- (P3HT-5F-N-CX) | additive [150] | morphology modulator, absorption range extender | P3HT:PCBM |
| P3HT-2Py-x | compatibilizer [137] | morphology modulator, photovoltaic activity modulator | P3HT:PCBM |
| P3HT-b-C60 | compatibilizer [148] | morphology modulator, thermal stress stabilizer | P3HT:PCBM |
| P3HT-b-P2VP | compatibilizer [135] | interface modulator, thermal stress stabilizer, mechanical stabilizer | P3HT:PCBM |
| P3HT-b-P2VP | additive [136] | interface modulator | P3HT:TiO2 |
| P3HT-b-P3PHT | additive [139] | morphology modulator, thermal stress stabilizer | P3HT:PCBM |
| P3HT-b-PS | compatibilizer [140] | morphology modulator | P3HT:PCBM |
| P3HT-b-PS | compatibilizer [141] | morphology modulator, interface stabilizer | P3HT:PCBM |
| P3HT-b-PS | compatibilizer [142] | morphology modulator, charges mobility booster | P3HT:PCBM |
| P3HT-b-PSFu | compatibilizer [147] | interface stabilizer | P3HT:PCBM |
| P3HT-b-PTMSM | additive [131] | passivation layer precursor | P3HT:PCBM |
| P3HT-b-P4VP | structuring agent [134] | interface modulator | P3HT:PCBM |
| PBDB-T | (ternary cell) [145] | morphology modulator | DRCN5T:PCBM |
| PBDTO-T | additive/dopant [144] | morphology modulator, charges mobility booster, energy levels matcher | PBDB-T:IT-M |
| PCBTDPP | (ternary cell) [143] | charges mobility booster, absorption range extender | P3HT:PCBM |
| PFLAM | additive [152] | charges mobility booster | P3HT:PCBM |
| Px | additive [132] | decreasing the rate of recombination processes | rr-P3HT:PCBM |
| PTB7-b-PNDI | additive [154] | morphology modulator | PTB7:PCBM |
| THC8 | additive [153] | morphology modulator, absorption range extender, charges mobility booster | P3HT:PCBM |
| CBs | Definition (according to the reference) | Major Role(s) | Compatibilized Blend |
|---|---|---|---|
| 1-chloronaphthalene | additive [170] | morphology modulator | PTVPhI-Eh:PCBM |
| 1-chloronaphthalene | additive [171] | morphology modulator | P3HT:PCBM |
| 1-chloronaphthalene | additive [172] | morphology modulator | P4T2F:PCBM |
| 1-chloronaphthalene | additive [173] | morphology modulator | PBDTTPD:PCBM |
| 3FMBPA | additive [179] | traps passivation | (PCDTBT:PCBM):ZnO |
| alkylthiophenes | additive [158] | morphology modulator | P3HT:PCBM |
| benzenes | additive [168] | morphology modulator | P3HT:PCBM |
| benzenes | additive [169] | morphology modulator | PTB7-Th:PC71BM |
| BT | surface modifier [180] | introducing a dipole at the interface. Boosting charges mobility | (PTB7-Th:PC71BM):ZnO |
| diphenyl ether | additive [166] | morphology modulator | BDT-QX:PCBM |
| diphenyl ether, 1-chloronaphthalene | additives [164] | morphology modulator, charges mobility booster | PBDB-T:m-ITIC |
| diphenyl ether, 1-chloronaphthalene | additives [165] | morphology modulator, absorption range extender | PTB7:PCBM |
| DTBT | additive [163] | morphology modulator, charges mobility booster | PtzBI/P(NDI2OD-T2) |
| F-DPE | additive [167] | morphology modulator | PTB7-Th:P(NDI2OD-T2) |
| FN-C60 | / [186] | morphology modulator, charges mobility booster | P3:PCBM |
| hydroxypyridines | additive [159] | morphology modulator, suppression of oxygen side-effects | P3HT:PCBM |
| hydroxypyridines | additive [161] | morphology modulator, thermal and air stability booster | PEDOT:PSS |
| hydroxypyridines | additive [162] | morphology modulator | PDPP3T:PCBM |
| IT-4F | (third component) [157] | morphology modulator, charges mobility booster | PBTA-PS:ITIC |
| naphthalenes | additive [174] | morphology modulator | P3HT:PCBM |
| naphthalenes | additive [175] | morphology modulator | PDTSTPD:/PCBM |
| PBD | dopant [181] | morphology modulator, decreasing work function, increasing conductivity | (PBDB-T:IT-M):ZnO |
| SA-x | additive [178] | morphology modulator, charges mobility booster | PBDB-TF:IT-4F |
| SH-na | additive [176] | morphology modulator, charges mobility booster | PTB7:PCBM |
| TDGTPA | electron-selective interlayer [156] | energy levels matcher | P3HT:PCBM |
| TiPS-pentacene | dopant [182] | energy levels matcher | P3HT:PCBM |
| xT-C60 | compatibilizer [185] | morphology modulator, interface stabilizer | P3HT:PCBM |
| xT-H-C60 | compatibilizer [187] | morphology modulator | P3HT:PCBM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonasera, A.; Giuliano, G.; Arrabito, G.; Pignataro, B. Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers. Molecules 2020, 25, 2200. https://doi.org/10.3390/molecules25092200
Bonasera A, Giuliano G, Arrabito G, Pignataro B. Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers. Molecules. 2020; 25(9):2200. https://doi.org/10.3390/molecules25092200
Chicago/Turabian StyleBonasera, Aurelio, Giuliana Giuliano, Giuseppe Arrabito, and Bruno Pignataro. 2020. "Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers" Molecules 25, no. 9: 2200. https://doi.org/10.3390/molecules25092200
APA StyleBonasera, A., Giuliano, G., Arrabito, G., & Pignataro, B. (2020). Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers. Molecules, 25(9), 2200. https://doi.org/10.3390/molecules25092200