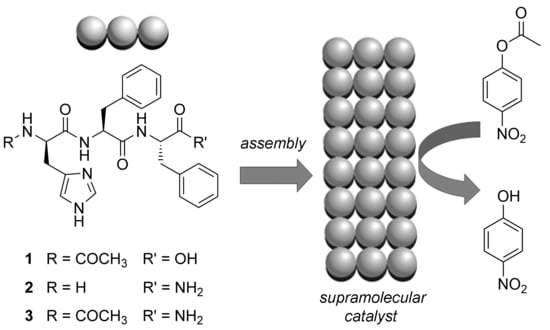
Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Self-Assembly into Nanostructured Hydrogels
2.2. Peptide Conformational Study
2.3. Esterase-Like Biocatalysis
3. Materials and Methods
3.1. Materials and General Methods
3.2. Oscillatory Rheology
3.3. Transmission Electron Microscopy
3.4. Circular Dichroism (CD)
3.5. Thioflavin T Fluorescence
3.6. ATR FT-IR Spectroscopy
3.7. Biocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, J.-M.; Han, S.-S.; Kim, H.-S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Smac Mimetics to Therapeutically Target IAP Proteins in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 157–169. [Google Scholar] [PubMed]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef] [PubMed]
- Nothling, M.D.; Xiao, Z.; Bhaskaran, A.; Blyth, M.T.; Bennett, C.W.; Coote, M.L.; Connal, L.A. Synthetic Catalysts Inspired by Hydrolytic Enzymes. ACS Catal. 2019, 9, 168–187. [Google Scholar] [CrossRef]
- Palocci, C.; Chronopoulou, L.; Venditti, I.; Cernia, E.; Diociaiuti, M.; Fratoddi, I.; Russo, M.V. Lipolytic Enzymes with Improved Activity and Selectivity upon Adsorption on Polymeric Nanoparticles. Biomacromolecules 2007, 8, 3047–3053. [Google Scholar] [CrossRef]
- Lu, Y.; Lv, Q.; Liu, B.; Liua, J. Immobilized Candida antarctica lipase B catalyzed synthesis of biodegradable polymers for biomedical applications. Biomater. Sci. 2019, 7, 4963–4983. [Google Scholar] [CrossRef]
- Szkolar, L.; Guilbaud, J.-B.; Miller, A.F.; Gough, J.E.; Saiani, A. Enzymatically triggered peptide hydrogels for 3D cell encapsulation and culture. J. Pept. Sci. 2014, 20, 578–584. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Su, L.; Li, Y.; Liu, Y.; An, Y.; Shi, L. Recent Advances and Future Prospects on Adaptive Biomaterials for Antimicrobial Applications. Macromol. Biosci. 2019, 19, e1900289. [Google Scholar] [CrossRef]
- Korendovych, I.V.; Dolan, M.A.; Korendovych, I.V. Catalytic peptide assemblies. Chem. Soc. Rev. 2018, 47, 3621–3639. [Google Scholar]
- Duncan, K.L.; Ulijn, R.V.; WestCHEMDepartment of Pure and Applied Chemistry University of Strathclyde Thomas Graham Building Cathedral Street Glasgow G XL UK. Advanced Science Research Center (ASRC)Hunter College City University of New York St Nicholas Terrace New York NY USA Short Peptides in Minimalistic Biocatalyst Design. Biocatalysis 2015, 1, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Stavrakoudis, A.; Makropoulou, S.; Tsikaris, V.; Sakarellos-Daitsiotis, M.; Sakarellos, C.; Demetropoulos, I.N. Computational screening of branched cyclic peptide motifs as potential enzyme mimetics. J. Pept. Sci. 2003, 9, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Makhlynets, O.V.; Matsui, H.; Korendovych, I.V. Design of Catalytic Peptides and Proteins Through Rational and Combinatorial Approaches. Annu. Rev. Biomed. Eng. 2016, 18, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, M.; Miravet, J.F.; Ulijn, R.V.; Escuder, B. Peptide-Based Molecular Hydrogels as Supramolecular Protein Mimics. Chem. Eur. J. 2017, 23, 981. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, X.; Luo, Q.; Li, Y.; Yang, K.; Zhuang, X.; Jiang, Y.; Zhang, J.; Liu, J.; Zou, G.; et al. Self-Assembled Peptide Nanofibers Designed as Biological Enzymes for Catalyzing Ester Hydrolysis. ACS Nano 2014, 8, 11715–11723. [Google Scholar] [CrossRef]
- Singh, N.; Conte, M.P.; Ulijn, R.V.; Miravet, J.F.; Escuder, B. Insight into the esterase like activity demonstrated by an imidazole appended self-assembling hydrogelator. Chem. Commun. 2015, 51, 13213–13216. [Google Scholar] [CrossRef] [Green Version]
- Gagni, P.; Romanato, A.; Bergamaschi, G.; Bettotti, P.; Vanna, R.; Piotto, C.; Morasso, C.F.; Chiari, M.; Cretich, M.; Gori, A. A self-assembling peptide hydrogel for ultrarapid 3D bioassays. Nanoscale Adv. 2019, 1, 490. [Google Scholar] [CrossRef] [Green Version]
- Akagawa, K.; Kudo, K. Solvolysis of Formylphenyl Esters by a Bifunctional Peptide Catalyst. Chem. Lett. 2016, 45, 300. [Google Scholar] [CrossRef]
- Carlomagno, T.; Cringoli, M.C.; Kralj, S.; Kurbasic, M.; Fornasiero, P.; Pengo, P.; Marchesan, S. Biocatalysis of D,L-Peptide Nanofibrillar Hydrogel. Molecules 2020, 25, 2995. [Google Scholar] [CrossRef]
- Song, R.; Wu, X.; Xue, B.; Yang, Y.; Huang, W.; Zeng, G.; Wang, J.; Li, W.; Cao, Y.; Wang, W.; et al. Principles Governing Catalytic Activity of Self-Assembled Short Peptides. J. Am. Chem. Soc. 2019, 141, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lei, B.; Wang, M.; Wu, S.; Qi, W.; Su, R.; He, Z. A supramolecular approach to construct a hydrolase mimic with photo-switchable catalytic activity. J. Mater. Chem. B 2018, 6, 2444. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Qi, W.; Su, R.; He, Z. Bioorganometallic ferrocene-tripeptide nanoemulsions. Nanoscale 2017, 9, 15323. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Kurbasic, M.; Kralj, S.; Melchionna, M.; Marchesan, S. A biocatalytic and thermoreversible hydrogel from a histidine-containing tripeptide. Chem. Commun. 2017, 53, 8110. [Google Scholar] [CrossRef] [PubMed]
- Kleinsmann, A.J.; Nachtsheim, B.J. A minimalistic hydrolase based on co-assembled cyclic dipeptides. Org. Biomol. Chem. 2020, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Rufo, C.M.; Moroz, Y.S.; Moroz, O.V.; Stohr, J.; Smith, T.A.; Hu, X.; DeGrado, W.F.; Korendovych, I.V. Short peptides self-assemble to produce catalytic amyloids. Nat. Chem. 2014, 6, 303. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.L.; Mikolajczak, D.J.; Böttcher, C.; Koksch, B. Substrate specificity of an actively assembling amyloid catalyst. Pept. Sci. 2017, 108, e23003. [Google Scholar] [CrossRef]
- Ting, Y.-H.; Chen, H.-J.; Cheng, W.-J.; Horng, J.-C. Zinc(II)–Histidine Induced Collagen Peptide Assemblies: Morphology Modulation and Hydrolytic Catalysis Evaluation. Biomacromolecules 2018, 19, 2629. [Google Scholar] [CrossRef]
- Makam, P.; Yamijala, S.S.R.K.C.; Tao, K.; Shimon, L.J.W.; Eisenberg, D.S.; Sawaya, M.R.; Wong, B.M.; Gazit, E. Non-proteinaceous hydrolase comprised of a phenylalanine metallo-supramolecular amyloid-like structure. Nat. Catal. 2019, 2, 977. [Google Scholar] [CrossRef]
- Gayen, K.; Basu, K.; Bairagi, D.; Castelletto, V.; Hamley, I.; Banerjee, A. An amino acid based metallo-hydrogel that acts like an esterase. ACS Appl. Bio Mater. 2018, 1, 1717. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Hao, S.; Han, A.; Yang, Y.; Luo, X.; Fang, G.; Liu, J.; Wang, S. Enzyme mimics based on self-assembled peptides for di(2-ethylhexyl)phthalate degradation. J. Mater. Chem. B 2020, 8, 9601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Hatfield, S.; Wan, R.; Zhu, Q.; Li, X.; McMills, M.; Ma, Y.; Li, J.; Brown, K.L.; et al. Dipeptide seryl-histidine and related oligopeptides cleave DNA, protein, and a carboxyl ester. Bioorg. Med. Chem. 2000, 8, 2675. [Google Scholar] [CrossRef]
- Gorlero, M.; Wieczorek, R.; Adamala, K.; Giorgi, A.; Schininà, M.E.; Stano, P.; Luisi, P.L. Ser-His catalyses the formation of peptides and PNAs. FEBS Lett. 2009, 583, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Guan, S.; Wang, Y.; Shi, G.; Cao, L.; Gao, Y.; Dong, Z.; Xu, J.; Luo, Q.; Liu, J. Self-assembly of amphiphilic peptides into bio-functionalized nanotubes: A novel hydrolase model. J. Mater. Chem. B 2013, 1, 2297. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Javid, N.; Duncan, K.; Birchall, L.; Gibson, K.F.; Cannon, D.; Kanetsuki, Y.; Knapp, C.; Tuttle, T.; Ulijn, R.V.; et al. Discovery of Catalytic Phages by Biocatalytic Self-Assembly. J. Am. Chem. Soc. 2014, 136, 15893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulseren, G.; Khalily, M.A.; Tekinay, A.B.; Guler, M.O. Catalytic supramolecular self-assembled peptide nanostructures for ester hydrolysis. J. Mater. Chem. B 2016, 4, 4605. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Romano, C.; Parisi, E.; Waddington, L.J.; Melchionna, M.; Semeraro, S.; De Zorzi, R.; Grönholm, M.; Marchesan, S. Bioadhesive supramolecular hydrogel from unprotected, short D,L-peptides with Phe-Phe and Leu-Asp-Val motifs. Chem. Commun. 2020, 56, 3015. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Byrne, T.; Woelfel, K.J.; Meany, J.E.; Spyridis, G.T.; Pocker, Y. The Hydrolysis of p-Nitrophenyl Acetate: A Versatile Reaction To Study Enzyme Kinetics. J. Chem. Educ. 1994, 71, 715. [Google Scholar] [CrossRef]
- Delort, E.; Nguyen-Trung, N.-Q.; Darbre, T.; Reymond, J.-L. Synthesis and Activity of Histidine-Containing Catalytic Peptide Dendrimers. J. Org. Chem. 2006, 71, 4468. [Google Scholar] [CrossRef]
- Cederholm, M.T.; Stuckey, J.A.; Doscher, M.S.; Lee, L. Histidine pKa shifts accompanying the inactivating Asp121—Asn substitution in a semisynthetic bovine pancreatic ribonuclease. Proc. Natl. Acad. Sci. USA 1991, 88, 8116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgcomb, S.; Murphy, K. Variability in the pKa of histidine side-chains correlates with burial within proteins. Proteins 2002, 49, 1. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.K.; Turner, G.J. Structural Basis of Perturbed pKa Values of Catalytic Groups in Enzyme Active Sites. IUBMB Life 2002, 53, 85. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Smith, A.M.; Collins, R.F.; Ulijn, R.V.; Saiani, A. Fmoc-diphenylalanine self-assembly mechanism induces apparent pKa shifts. Langmuir 2009, 25, 9447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shafi, R.; Lampel, A.; MacPherson, D.; Pappas, C.G.; Narang, V.; Wang, T.; Maldarelli, C.; Ulijn, R.V. Switchable Hydrolase Based on Reversible Formation of Supramolecular Catalytic Site Using a Self-Assembling Peptide. Angew. Chem. Int. Ed. 2017, 56, 14511. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.M.; Doran, T.M.; Anderson, S.B.; Nilsson, B.L. Effect of C-Terminal Modification on the Self-Assembly and Hydrogelation of Fluorinated Fmoc-Phe Derivatives. Langmuir 2011, 27, 4029. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Melle-Franco, M.; Kurbasic, M.; Melchionna, M.; Abrami, M.; Grassi, M.; Prato, M.; Marchesan, S. Oxidized Nanocarbons-Tripeptide Supramolecular Hydrogels: Shape Matters! ACS Nano 2018, 12, 5530. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Iglesias, D.; Parisi, E.; Styan, K.E.; Waddington, L.J.; Deganutti, C.; De Zorzi, R.; Grassi, M.; Melchionna, M.; Vargiu, A.V.; et al. Chirality Effects on Peptide Self-Assembly Unraveled from Molecules to Materials. Chem 2018, 4, 1862. [Google Scholar] [CrossRef] [Green Version]
- Marchesan, S.; Styan, K.E.; Easton, C.D.; Waddington, L.; Vargiu, A.V. Higher and lower supramolecular orders for the design of self-assembled heterochiral tripeptide hydrogel biomaterials. J. Mater. Chem. B 2015, 3, 8123. [Google Scholar] [CrossRef] [Green Version]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Biancalana, M.; Koide, S.; Shea, J.E. Binding modes of thioflavin-T to the single-layer beta-sheet of the peptide self-assembly mimics. J. Mol. Biol. 2009, 394, 627. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Erez, Y.; Huppert, D. Molecular rotors: What lies behind the high sensitivity of the thioflavin-T fluorescent marker. Acc. Chem. Res. 2012, 45, 1548. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bowers, M.T.; Shea, J.E. On the origin of the stronger binding of PIB over thioflavin T to protofibrils of the Alzheimer amyloid-beta peptide: A molecular dynamics study. Biophys. J. 2011, 100, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Tripeptide | N-Terminus | C-Terminus | 1 mM | 25 mM | 50 mM |
|---|---|---|---|---|---|
| l-His–d-Phe–d-Phe | NH2 | COOH | Sol | Sol | Hydrogel |
| 1 | Acetylated | COOH | Sol | Sol | Aggregates |
| 2 | NH2 | Amidated | Sol | Hydrogel | Hydrogel |
| 3 | Acetylated | Amidated | Sol | Hydrogel | Hydrogel |
| [pNPA] mM | 1 (Sol) | 1 (Fibrils) | 1 (Aggregates) | 2 (Hydrogel) | 3 (Hydrogel) |
|---|---|---|---|---|---|
| 0.2 | 1.02 × 10−5 | 2.17 × 10−4 | 1.05 × 10−4 | 3.55 × 10−4 | 3.83 × 10−5 |
| 0.4 | 1.65 × 10−5 | 4.39 × 10−4 | 2.00 × 10−4 | 2.60 × 10−3 | 1.50 × 10−4 |
| 0.6 | 2.43 × 10−5 | 7.61 × 10−4 | 3.57 × 10−4 | 5.00 × 10−3 | 3.11 × 10−4 |
| 0.8 | 3.02 × 10−5 | 9.70 × 10−4 | 4.32 × 10−4 | 7.26 × 10−3 | 4.41 × 10−4 |
| 1.0 | 4.43 × 10−5 | 1.16 × 10−3 | 6.60 × 10−4 | 8.68 × 10−3 | 4.55 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbasic, M.; Garcia, A.M.; Viada, S.; Marchesan, S. Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis. Molecules 2021, 26, 173. https://doi.org/10.3390/molecules26010173
Kurbasic M, Garcia AM, Viada S, Marchesan S. Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis. Molecules. 2021; 26(1):173. https://doi.org/10.3390/molecules26010173
Chicago/Turabian StyleKurbasic, Marina, Ana M. Garcia, Simone Viada, and Silvia Marchesan. 2021. "Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis" Molecules 26, no. 1: 173. https://doi.org/10.3390/molecules26010173
APA StyleKurbasic, M., Garcia, A. M., Viada, S., & Marchesan, S. (2021). Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis. Molecules, 26(1), 173. https://doi.org/10.3390/molecules26010173