Synthesis and Antiproliferatory Activities Evaluation of Multi-Substituted Isatin Derivatives
Abstract
:1. Introduction
2. Results and Discussion
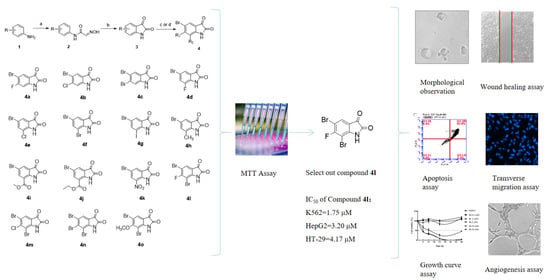
2.1. Synthesis of Compounds 4a-4o
2.2. Cytotoxic Effect Against K562 and HepG2 Treated with Compound 4l In Vitro
2.3. Cell Proliferation Assay of Compound 4l
2.4. Morphological Changes of K562 Cells Treated with Compound 4l
2.5. Compound 4l Inhibited K562 Cell Apoptosis
2.6. Compound 4l Inhibited K562 Cell Proliferation
2.7. Compound 4l Suppressed Cell Migration in HepG2 Cells and Tube Formation in HUVECs
2.8. Compound 4l Suppressed Tube Formation in HUVECs
3. Conclusions
4. Materials and Methods
4.1. General
4.1.1. General Procedure I: Synthesis of Compound 3
4.1.2. General Procedure II: Synthesis of Multi-Substituted Isatin Derivatives (4a–4c)
4.1.3. General Procedure III: Synthesis of Multi-Substituted Isatin Derivatives (4d–4j)
4.1.4. General Procedure IV: Synthesis of Multi-Substituted Isatin Derivatives (4l–4o)
4.2. Biology
4.2.1. Cell Lines and Culture Conditions
4.2.2. MTT Assay for Cytotoxicity
4.2.3. Apoptosis Analysis
4.2.4. Cell Growth Curve Experiment
4.2.5. Wound Healing Assay
4.2.6. Transwell Assay
4.2.7. Tube Forming Assay
4.2.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Medvedev, A.E.; Buneeva, O.A.; Kopylov, A.T.; Tikhonova, O.V.; Medvedeva, M.V.; Nerobkova, L.N.; Kapitsa, I.G.; Zgoda, V.G. Brain Mitochondrial Subproteome of Rpn10-Binding Proteins and Its Changes Induced by the Neurotoxin MPTP and the Neuroprotector Isatin. Biochemistry 2017, 82, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, P.; Kashanian, S.; Khodaei, M.M.; Harding, F.J. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacol. Rep. 2013, 65, 313–335. [Google Scholar] [CrossRef]
- Song, J.; Hou, L.; Ju, C.; Zhang, J.; Ge, Y.; Yue, W. Isatin inhibits proliferation and induces apoptosis of SH-SY5Y neuroblastoma cells in vitro and in vivo. Eur. J. Pharm. 2013, 702, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological activities of isatin and its derivatives. Acta Pharm 2005, 55, 27–46. [Google Scholar] [PubMed]
- Cane, A.; Tournaire, M.C.; Barritault, D.; Crumeyrolle-Arias, M. The endogenous oxindoles 5-hydroxyoxindole and isatin are antiproliferative and proapoptotic. Biochem. Biophys. Res. Commun. 2000, 276, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Igosheva, N.; Lorz, C.; O’Conner, E.; Glover, V.; Mehmet, H. Isatin, an endogenous monoamine oxidase inhibitor, triggers a dose- and time-dependent switch from apoptosis to necrosis in human neuroblastoma cells. Neurochem. Int. 2005, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Nagarsenkar, A.; Guntuku, L.; Guggilapu, S.D.; Danthi, B.K.; Gannoju, S.; Naidu, V.G.M.; Bathini, N.B. Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. Eur. J. Med. Chem. 2016, 124, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, L.; Hou, L.; Ju, C.; Zhao, S.; Wei, Y. Isatin inhibits SH-SY5Y neuroblastoma cell invasion and metastasis through MAO/HIF-1alpha/CXCR4 signaling. Anticancer Drugs 2017, 28, 645–653. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Nguewa, P.A.; Redrado, M.; Manrique, I.; Calvo, A. Sunitinib reduces tumor hypoxia and angiogenesis, and radiosensitizes prostate cancer stem-like cells. Prostate 2015, 75, 1137–1149. [Google Scholar] [CrossRef]
- Atkins, M.; Jones, C.A.; Kirkpatrick, P. Sunitinib maleate. Nat. Rev. Drug Discov. 2006, 5, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E.; Yu, B.; Rice, A.; Lipson, K.E.; Liang, C.; Sun, L.; Tang, C.; McMahon, G.; Pestell, R.G.; Wadler, S. A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001, 61, 6170–6177. [Google Scholar] [PubMed]
- Gao, N.; Kramer, L.; Rahmani, M.; Dent, P.; Grant, S. The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism. Mol. Pharm. 2006, 70, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Jansa, J.; Lyčka, A.; Růžička, A.; Grepl, M.; Vaněček, J. Synthesis, structure and rearrangement of iodinated imidazo[1,2- c]pyrimidine-5(6 H)-ones derived from cytosine. Tetrahedron 2015, 71, 27–36. [Google Scholar] [CrossRef]
- Prabhakara, C.T.; Patil, S.A.; Toragalmath, S.S.; Kinnal, S.M.; Badami, P.S. Synthesis, characterization and biological approach of metal chelates of some first row transition metal ions with halogenated bidentate coumarin Schiff bases containing N and O donor atoms. J. Photochem. Photobiol. B 2016, 157, 1–14. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Liu, H.; Zheng, L.; Tong, Y.; Qu, D.; Han, S. Biological evaluation of halogenated thiazolo[3,2-a]pyrimidin-3-one carboxylic acid derivatives targeting the YycG histidine kinase. Eur. J. Med. Chem. 2014, 87, 500–507. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany Ny) 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Teng, Y.; Yuan, Y.; Ruan, T.T.; Wang, Q.; Gao, X.; Zhou, Y.; Han, K.L.; Yu, P.; Lu, K. Synthesis and cytotoxic studies of novel 5-phenylisatin derivatives and their anti-migration and anti-angiogenic evaluation. Eur. J. Med. Chem. 2018, 156, 800–814. [Google Scholar] [CrossRef]
- Chen, H.Q.; Xing, Y.J.; Xie, J.; Xie, J.Q.; Xing, D.; Tang, J.; Yang, F.; Yi, Z.F.; Qiu, W.W. Synthesis and biological evaluation of 3-nitro-4-chromanone derivatives as potential antiproliferative agents for castration-resistant prostate cancer. Rsc Adv. 2019, 9, 33794–33799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fu, Y.; Zhao, Y.F.; Cui, S.S.; Wang, J.; Liu, F.X.; Yuan, Y.; Galons, H.; Yu, P.; Teng, Y. 5-Acetamido-1-(methoxybenzyl) isatin inhibits tumor cell proliferation, migration, and angiogenesis. Rsc Adv. 2019, 9, 36690–36698. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.S.; Yuan, Y.; Zhang, Q.; Rong, Y.; Yang, Y.; Chi, M.; Liu, Z.; Zhang, Y.M.; Yu, P.; Teng, Y.U. Effects of an isatin derivative on tumor cell migration and angiogenesis. Rsc Adv. 2020, 10, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.O.; Zhao, H.Y.; Wang, J.; Liu, H.; Gao, M.L.; Zhou, Y.; Han, K.L.; Fan, Z.C.; Zhang, Y.M.; Sun, H.; et al. Synthesis and anti-cancer activity evaluation of 5-(2-carboxyethenyl)-isatin derivatives. Eur. J. Med. Chem. 2016, 112, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, Y.; Fujii, T.; Saito, H.; Sano, M.; Uchiyama, T.; Miyairi, S. 5-Bromoindirubin 3’-(O-oxiran-2-ylmethyl)oxime: A long-acting anticancer agent and a suicide inhibitor for epoxide hydrolase. Bioorg. Med. Chem. 2017, 25, 4665–4676. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.V.; Esteves, P.M.; Pinto, A.C. Chlorination of Isatins with Trichloroisocyanuric Acid. J. Braz. Chem. Soc. 2011, 22, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Benkendorff, K.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931. [Google Scholar] [CrossRef] [PubMed]





| Compound | IC50 (µM) a | ||
|---|---|---|---|
| K562 | HepG2 | HT-29 | |
| CPTb | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| 3f | >10 | >10 | >10 |
| 4a | 2.32 ± 0.22 | 22.93 ± 2.18 | 15.26 ± 0.24 |
| 4b | 15.16 ± 1.63 | 28.22 ± 2.06 | 31.93 ± 1.37 |
| 4c | 12.75 ± 1.38 | 26.07 ± 2.15 | 2.14 ± 0.15 |
| 4d | 30.22 ± 0.52 | >100 | >100 |
| 4e | 11.14 ± 1.23 | 14.45 ± 1.36 | 30.83 ± 0.80 |
| 4f | 21.36 ± 2.58 | 23.52 ± 2.01 | 2.67 ± 0.20 |
| 4g | 23.38 ± 1.57 | 30.68 ± 2.39 | 31.78 ± 1.21 |
| 4h | 20.27 ± 2.02 | >100 | >100 |
| 4i | 34.61 ± 0.92 | 33.74 ± 1.06 | 26.11 ± 0.32 |
| 4j | 73.53 ± 4.16 | >100 | 93.2 ± 2.01 |
| 4k | >100 | >100 | >100 |
| 4l | 1.75 ± 0.16 | 3.20 ± 0.14 | 4.17 ± 0.33 |
| 4m | 5.05 ± 0.48 | 36.93 ± 1.26 | 37.26 ± 0.60 |
| 4n | 2.85 ± 0.95 | 33.34 ± 1.92 | 3.68 ± 0.32 |
| 4o | 34.24 ± 0.58 | 52.70 ± 3.20 | 42.84 ± 2.98 |
| Cell Line | IC50 (μM) | |
|---|---|---|
| Compound 4l | CPT | |
| Human leukemia (K562) | 1.75 ± 0.16 | 0.03 ± 0.01 |
| Liver cancer (HepG2) | 3.20 ± 0.14 | 0.04 ± 0.01 |
| Human Colon cancer (HT-29) | 4.17 ± 0.33 | 0.06 ± 0.01 |
| Colon cancer (HCT-116) | 6.18 ± 0.22 | 0.24 ± 0.07 |
| Cancer cells (MDA-MB-231) | >100 | >100 |
| Human Prostate cancer (PC-3) | >100 | >100 |
| Lung cancer (A549) | 18.94 ± 1.03 | >100 |
| Renal epithelial cells (293T) | >100 | 9.84 ± 1.81 |
| Umbilical vein endothelial cells (HUVEC) | 61.83 ± 2.24 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zhao, L.; Fu, Y.; Hao, L.; Fu, Y.; Yuan, Y.; Yu, P.; Teng, Y. Synthesis and Antiproliferatory Activities Evaluation of Multi-Substituted Isatin Derivatives. Molecules 2021, 26, 176. https://doi.org/10.3390/molecules26010176
Ding Y, Zhao L, Fu Y, Hao L, Fu Y, Yuan Y, Yu P, Teng Y. Synthesis and Antiproliferatory Activities Evaluation of Multi-Substituted Isatin Derivatives. Molecules. 2021; 26(1):176. https://doi.org/10.3390/molecules26010176
Chicago/Turabian StyleDing, Ying, Lianbo Zhao, Ying Fu, Lei Hao, Yupeng Fu, Yuan Yuan, Peng Yu, and Yuou Teng. 2021. "Synthesis and Antiproliferatory Activities Evaluation of Multi-Substituted Isatin Derivatives" Molecules 26, no. 1: 176. https://doi.org/10.3390/molecules26010176
APA StyleDing, Y., Zhao, L., Fu, Y., Hao, L., Fu, Y., Yuan, Y., Yu, P., & Teng, Y. (2021). Synthesis and Antiproliferatory Activities Evaluation of Multi-Substituted Isatin Derivatives. Molecules, 26(1), 176. https://doi.org/10.3390/molecules26010176