Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure Analysis
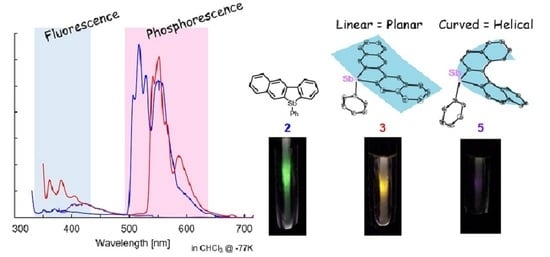
2.3. Optical Properties
3. Materials and Methods
3.1. General Information
3.2. Preparation and Characterization of Novel Compounds
3.2.1. 2-Bromo-3-(2-bromophenyl)naphthalene (1a)
3.2.2. 5-Phenylbenzo[b]naphtho[2,3-d]stibole (2)
3.2.3. 6-Phenyldinaphtho[2,3-b,2′,3′-d]stibole (3)
3.2.4. 6-Phenyldinaphtho[2,3-b,2′,3′-d]arsole (4)
3.2.5. 7-Phenyldinaphtho[2,1-b,1′,2′-d]stibole (5)
3.3. Single Crystal X-ray Diffraction Experiment
3.3.1. Compound 3
3.3.2. Compound 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baumgartner, T.; Jäkle, F. (Eds.) Main Group Strategies towards Functional Hybrid Materials; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Hissler, M.; Dyer, P.W.; Réau, R. Linear organic π-conjugated systems featuring the heavy Group 14 and 15 elements. Coord. Chem. Rev. 2003, 244, 1–44. [Google Scholar] [CrossRef]
- Baumgartner, T.; Réau, R. Organophosphorus π-conjugated materials. Chem. Rev. 2006, 106, 4681–4727. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.G.; Baumgartner, T. Recent developments in phosphole-containing oligo- and polythiophene materials. Eur. J. Inorg. Chem. 2007, 3611–3628. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Bezkishko, I.A.; Miluykov, V.A.; Sinyashin, O.G. Phospholes-development and recent advances. Mendeleev Commun. 2013, 23, 117–130. [Google Scholar] [CrossRef]
- Duffy, M.P.; Delaunay, W.; Bouit, P.-A.; Hissler, M. π-Conjugated phospholes and their incorporation into devices: Components with a great deal of potential. Chem. Soc. Rev. 2016, 45, 5296–5310. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, K.; Tanaka, S.; Imoto, H.; Naka, K. 2-Arylbenzo[b]arsoles: An experimental and computational study on the relationship between structural and photophysical properties. Dalton Trans. 2020, 49, 15612–15621. [Google Scholar] [CrossRef]
- Fujii, T.; Tanaka, S.; Hayashi, S.; Imoto, H.; Naka, K. Dipyridinoarsole: A new class of stable and modifiable heteroatom-bridged bipyridines. Chem. Commun. 2020, 56, 6035–6038. [Google Scholar] [CrossRef]
- Urushizaki, A.; Yumura, T.; Kitagawa, Y.; Hasegawa, Y.; Imoto, H.; Naka, K. Dithieno[3,4-b:3′,4′-d]arsole: A novel class of hetero[5]radialenes. Eur. J. Org. Chem. 2020, 2020, 3965–3970. [Google Scholar] [CrossRef]
- Tanaka, S.; Enoki, T.; Imoto, H.; Ooyama, Y.; Ohshita, J.; Kato, T.; Naka, K. Highly efficient singlet oxygen generation and high oxidation resistance enhanced by arsole-polymer-based photosensitizer: Application as a recyclable photooxidation catalyst. Macromolecules 2020, 53, 2006–2013. [Google Scholar] [CrossRef]
- Imoto, H.; Naka, K. The Dawn of functional organoarsenic chemistry. Chem. Eur. J. 2019, 25, 1883–1894. [Google Scholar] [CrossRef]
- Rivard, E. Tellurophenes and their emergence as building blocks for polymeric and light-emitting materials. Chem. Lett. 2015, 44, 730–736. [Google Scholar] [CrossRef]
- Parke, S.M.; Narreto, M.A.B.; Hupf, E.; McDonald, R.; Ferguson, M.J.; Hegmann, F.A.; Rivard, E. Understanding the origin of phosphorescence in bismoles: A synthetic and computational study. Inorg. Chem. 2018, 57, 7536–7549. [Google Scholar] [CrossRef] [PubMed]
- Parke, S.M.; Hupf, E.; Matharu, G.K.; de Aguiar, I.; Xu, L.; Yu, H.; Boone, M.P.; de Souza, G.L.C.; McDonald, R.; Ferguson, M.J.; et al. Aerobic solid state red phosphorescence from benzobismole monomers and patternable self-assembled block copolymers. Angew. Chem. Int. Ed. 2018, 57, 14841–14846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, J.; Liu, B.; Tan, Q.; Xu, B. Synthesis of tellurium-containing π-extended aromatics with room-temperature phosphorescence. Org. Lett. 2019, 21, 8328–8333. [Google Scholar] [CrossRef] [PubMed]
- Hupf, E.; Tsuchiya, Y.; Moffat, W.; Xu, L.; Hirai, M.; Zhou, Y.; Ferguson, M.J.; McDonald, R.; Murai, T.; He, G.; et al. A modular approach to phosphorescent π-extended heteroacenes. Inorg. Chem. 2019, 58, 13323–13336. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L.; Berman, J.D. Recommendations for treating Leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am. J. Trop. Med. Hyg. 1992, 46, 296–306. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mitani, M.; Yasuike, S.; Kurita, J.; Kaji, T. An organobismuth compound that exhibits selective cytotoxicity to vascular endothelial cells in vitro. J. Health Sci. 2005, 51, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Kohri, K.; Yoshida, E.; Yasuike, S.; Fujie, T.; Yamamoto, C.; Kaji, T. The cytotoxicity of organobismuth compounds with certain molecular structures can be diminished by replacing the bismuth atom with an antimony atom in the molecules. J. Toxicol. Sci. 2015, 40, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Nakano, S.; Kitamura, Y.; Yamamoto, C.; Yasuike, S.; Kaji, T. Intracellular accumulation-independent cytotoxicity of pentavalent organoantimony compounds in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Sum, H. (Ed.) Biological Chemistry of Aresenic, Antimony and Bismuth; Jhon Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Ashe, A.J.; Drone, F.J. Synthesis of 1-phenylarsole and 1-phenylstibole. Organometallics 1985, 4, 1478–1480. [Google Scholar] [CrossRef]
- Kurita, J.; Ishii, M.; Yasuike, S.; Tsuchiya, T. A versatile synthetic route to 1-benzometalloles involving the first examples of several C-unsubstituted benzometalloles. J. Chem. Soc. Chem. Commun. 1993, 1309–1310. [Google Scholar] [CrossRef]
- Kurita, J.; Ishii, M.; Yasuike, S.; Tsuchiya, T. Synthesis of 1-benzometalloles containing group 14, 15, and 16 heavier elements via a common dilithiostyrene intermediate. Chem. Pharm. Bull 1994, 42, 1437–1441. [Google Scholar] [CrossRef] [Green Version]
- Heinekey, D.M.; Millar, I.T. The synthesis of 9-alkyl- or 9-aryl-9-arsafluorenes or -9-stibiafluorenes. J. Chem. Soc. 1959, 3101–3102. [Google Scholar]
- Kurita, J.; Usuda, F.; Yasuike, S.; Tsuchiya, T.; Tsuda, Y.; Kiuchi, F.; Hosoi, S. Resolution of racemic Sb-chiral stibindoles using an optically active ortho-palladated benzylamine derivative, via their diastereomeric complexes. Chem. Commun. 2000, 191–192. [Google Scholar] [CrossRef]
- Christianson, A.M.; Rivard, E.; Gabbaï, F.P. 1λ5-stibaindoles as lewis acidic, π-conjugated, fluoride anion responsive platforms. Organometallics 2017, 36, 2670–2676. [Google Scholar] [CrossRef]
- Christianson, A.M.; Gabbaï, F.P. A lewis acidic, π-conjugated stibaindole with a colorimetric response to anion binding at Sb(III). Organometallics 2017, 36, 3013–3015. [Google Scholar] [CrossRef]
- Ohshita, J.; Fujita, R.; Tanaka, D.; Ooyama, Y.; Kobayashi, N.; Higashimura, H.; Yamamoto, Y. Synthesis and optical properties of dithienostiboles. Chem. Lett. 2012, 41, 1002–1003. [Google Scholar] [CrossRef]
- Ohshita, J.; Yamaji, K.; Ooyama, Y.; Adachi, Y.; Nakamura, M.; Watase, S. Synthesis, properties, and complex formation of antimony- and bismuth-bridged bipyridyls. Organometallics 2019, 38, 1516–1523. [Google Scholar] [CrossRef]
- Yasuike, S.; Iida, T.; Yamaguchi, K.; Seki, H.; Kurita, J. Synthesis, molecular structure and fluxional behavior of (R)-7-p-tolyldinaphtho[2,1-b;1′,2′-d]stibole: The first isolated example of optically active group 15 dinaphthoheteroles. Tetrahedron Lett. 2001, 42, 441–444. [Google Scholar] [CrossRef]
- Matsumura, M.; Kawahata, M.; Muranaka, A.; Hiraiwa, M.; Yamaguchi, K.; Uchiyama, M.; Yasuike, S. Efficient synthesis, structural Characterization, and optical properties of 6H-dibenzo[b,h]carbazole and its derivatives. Eur. J. Org. Chem. 2019, 3788–3793. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates and Resolutions; Krieger Publishing Company: Malabar, FL, USA, 1991. [Google Scholar]
- Wang, Y.; Chen, A.M. Enantioenrichment by crystallization. Org. Process Res. Dev. 2008, 12, 282–290. [Google Scholar] [CrossRef]
- Buchwald, S.L.; Fisher, R.A.; Foxman, B.M. The synthesis and structure of stibaindoles. Angew. Chem. Int. Ed. 1990, 29, 771–772. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-F.; Shen, Y. Helicene Chemistry: From Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. 2015, C71, 3–8. [Google Scholar]





| Compound 3 | Compound 5 | ||
|---|---|---|---|
| Bond length (Å) | C(1)-Sb(1) | 2.182(9) | 2.158(2) |
| C(2)-Sb(1) | 2.155(10) | 2.138(2) | |
| C(3)-Sb(1) | 2.171(8) | 2.146(2) | |
| Bond angle (°) | C(2)-Sb(1)-C(3) | 81.0(3) | 80.03(8) |
| C(1)-Sb(1)-C(2) | 92.7(3) | 100.33(8) | |
| C(1)-Sb(1)-C(3) | 94.6(3) | 89.47(8) | |
| Torsion angle (°) | C(4)-C(5)-C(6)-C(7) | 0.0(13) | 36.3(3) |
| Compd. | UV λabs (ε) (nm) | λflb | Φflb,c | λphosd | τphosd |
|---|---|---|---|---|---|
| (nm) | (%) | (nm) | (ms) | ||
| 2 | 268 (29500), 323 (11300) | 356, 373 | 1.0 | 517, 529 | 40 |
| 3 | 288 (54100), 340 (20700), 360 (3700) | 365, 385 | 0.5 | 551, 588 | 45 |
| 4 | 266 (84800), 341 (15300), 358 (6100) | 362 | 3.0 | 537, 583 | 205 |
| 5 | 363 (12200) | 406.5 | 0.03 | n.d. e | n.d. e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, M.; Matsuhashi, Y.; Kawakubo, M.; Hyodo, T.; Murata, Y.; Kawahata, M.; Yamaguchi, K.; Yasuike, S. Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules 2021, 26, 222. https://doi.org/10.3390/molecules26010222
Matsumura M, Matsuhashi Y, Kawakubo M, Hyodo T, Murata Y, Kawahata M, Yamaguchi K, Yasuike S. Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules. 2021; 26(1):222. https://doi.org/10.3390/molecules26010222
Chicago/Turabian StyleMatsumura, Mio, Yuki Matsuhashi, Masato Kawakubo, Tadashi Hyodo, Yuki Murata, Masatoshi Kawahata, Kentaro Yamaguchi, and Shuji Yasuike. 2021. "Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles" Molecules 26, no. 1: 222. https://doi.org/10.3390/molecules26010222
APA StyleMatsumura, M., Matsuhashi, Y., Kawakubo, M., Hyodo, T., Murata, Y., Kawahata, M., Yamaguchi, K., & Yasuike, S. (2021). Synthesis, Structural Characterization, and Optical Properties of Benzene-Fused Tetracyclic and Pentacyclic Stiboles. Molecules, 26(1), 222. https://doi.org/10.3390/molecules26010222