Cattleianal and Cattleianone: Two New Meroterpenoids from Psidium cattleianum Leaves and Their Selective Antiproliferative Action against Human Carcinoma Cells
Abstract
:1. Introduction
2. Results
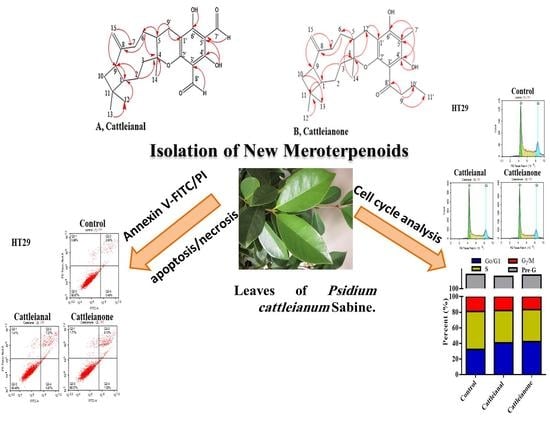
2.1. Identification of the Isolated Compounds
2.2. Cytotoxicity Assessment of Cattleianal and Cattleianone
2.3. Influence of Cattleianal and Cattleianone on Cell Cycle Distribution
2.4. Apoptosis/Necrosis Assessment Using Flow Cytometry
2.5. Molecular Assessment of Apoptosis
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. Cytotoxicity Assays
4.6. Cell Cycle Analysis
4.7. Apoptosis Assessment
4.8. Apoptosis Gene Expression Analysis
4.9. Assessment of Active Caspase-3 Concentration
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Saber, F.R.; Ashour, R.M.; El-Halawany, A.M.; Mahomoodally, M.F.; Ak, G.; Zengin, G.; Mahrous, E.A. Phytochemical profile, enzyme inhibition activity and molecular docking analysis of Feijoa sellowiana O. Berg. J. Enzym. Inhib. Med. Chem. 2021, 36, 618–626. [Google Scholar] [CrossRef]
- Ismail, M.M.; Samir, R.; Saber, F.R.; Ahmed, S.R.; Farag, M.A. Pimenta Oil as A potential treatment for Acinetobacter Baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics 2020, 9, 679. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from Guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef] [Green Version]
- Faleiro, J.H.; Gonçalves, R.C.; Dos Santos, M.N.G.; Da Silva, D.P.; Naves, P.L.F.; Malafaia, G. The Chemical Featuring, Toxicity, and Antimicrobial activity of Psidium cattleianum (Myrtaceae) leaves. New J. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Patel, S. Exotic tropical plant Psidium cattleianum: A review on prospects and threats. Rev. Environ. Sci. Bio/Technol. 2012, 11, 243–248. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; Cardoso, J.D.S.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and characterization of phytochemical compounds from araçazeiro (Psidium cattleianum) leaf: Putative antioxidant and antimicrobial properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef]
- dos Santos Pereira, E.; Vinholes, J.; Franzon, R.C.; Dalmazo, G.; Vizzotto, M.; Nora, L. Psidium cattleianum fruits: A review on its composition and bioactivity. Food Chem. 2018, 258, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.Y.; Mosaddik, A.; Kim, H.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Cho, S.K. The chloroform fraction of guava (Psidium cattleianum sabine) leaf extract inhibits human gastric cancer cell proliferation via induction of apoptosis. Food Chem. 2011, 125, 369–375. [Google Scholar] [CrossRef]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-C.; Peng, C.-C.; Chiu, W.-T.; Cheng, Y.-T.; Huang, G.-T.; Hsieh, C.-L.; Peng, R.Y. Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr. Cancer 2010, 62, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-J.; Yu, Q.; Yan, H.; Khan, A.; Feng, M.-Y.; Li, P.-P.; Hao, X.-J.; An, L.-K.; Liu, H.-Y. Meroterpenoids with anti-tumor activities from guava (Psidium guajava). J. Agric. Food Chem. 2017, 65, 4993–4999. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, H.-L.; Zhang, J.; Liu, W.-Y.; Luo, J.-G.; Pan, K.; Cao, W.-Y.; Bi, Q.-R.; Feng, F.; Qu, W. Littordials A–E, novel formyl-phloroglucinol-β-caryophyllene meroterpenoids from the leaves of Psidium littorale. Org. Chem. Front. 2019, 6, 1667–1673. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Hu, Y.-W.; Qu, W.; Zhang, J.; Guo, E.-Y.; Jiang, X.-Y.; Liu, W.-Y.; Feng, F.; Xu, J.; Littordial, F. A novel phloroglucinol meroterpenoid from the leaves of Psidium littorale. Tetrahedron Lett. 2019, 60, 1868–1870. [Google Scholar] [CrossRef]
- Yang, X.-L.; Hsieh, K.-L.; Liu, J.-K. Guajadial: An unusual meroterpenoid from Guava leaves Psidium guajava. Org. Lett. 2007, 9, 5135–5138. [Google Scholar] [CrossRef]
- Fu, H.-Z.; Luo, Y.-M.; Li, C.-J.; Yang, J.-Z.; Zhang, D.-M.; Psidials, A.-C. Three unusual meroterpenoids from the leaves of Psidium guajava L. Org. Lett. 2010, 12, 656–659. [Google Scholar] [CrossRef]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Chang, C.-S.; Yeh, W.-L.; Tang, C.-H.; Cheung, C.-W.; Leung, Y.-M.; Liu, J.-F.; Wong, K.-L. The novel phloroglucinol derivative BFP induces apoptosis of glioma cancer through reactive oxygen species and endoplasmic reticulum stress pathways. Phytomedicine 2012, 19, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.A.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Al-Abd, A.M.; Saber, F.R.; El-Halawany, A.M. Cytotoxic activity of acyl phloroglucinols isolated from the leaves of Eucalyptus cinerea F. Muell. ex Benth. cultivated in Egypt. Sci. Rep. 2014, 4, 5410. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, J.; Lock, R.; Liu, T. Tumor protein 53-induced nuclear protein 1 enhances p53 function and represses tumorigenesis. Front. Genet. 2013, 4, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Sidana, J.; Rohilla, R.K.; Roy, N.; Barrow, R.A.; Foley, W.J.; Singh, I.P. Antibacterial sideroxylonals and loxophlebal A from Eucalyptus loxophleba foliage. Fitoterapia 2010, 81, 878–883. [Google Scholar] [CrossRef]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; AlShehri, O.Y.; Al-Abd, A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef] [Green Version]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Bashmail, H.A.; AlAmoudi, A.A.; Noorwali, A.; Hegazy, G.A.; Ajabnoor, G.; Choudhry, H.; Al-Abd, A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018, 8, 11674. [Google Scholar] [CrossRef] [Green Version]




| Compound | HFB4 | HepG2 | HEp2 | MCF7 | HCT-116 | HT-29 | MDA-MB-231 |
|---|---|---|---|---|---|---|---|
| Cattleianal (1) | >100 | 40.3 ± 1.35 | 28.3 ± 0.1 | 44.8 ± 2.2 | >100 | 35.2 ± 4.2 | 64.4 ± 5.6 |
| Cattleianone (2) | >100 | 22.0 ± 0.9 | 33.6 ± 2.8 | 23.7 ± 1.6 | 70.5 ± 1.6 | 32.1 ± 1.4 | 38.7 ± 3.5 |
| Doxorubicin | 7.36 ± 0.33 | 7.73 ± 0.8 | 8.0 ± 0.9 | 7.73 ± 0.6 | 0.76 ± 0.1 | 0.78 ± 0.1 | 0.83 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Mahrous, E.; Al-Abd, A.M.; Salama, M.M.; Fathy, M.M.; Soliman, F.M.; R. Saber, F. Cattleianal and Cattleianone: Two New Meroterpenoids from Psidium cattleianum Leaves and Their Selective Antiproliferative Action against Human Carcinoma Cells. Molecules 2021, 26, 2891. https://doi.org/10.3390/molecules26102891
A. Mahrous E, Al-Abd AM, Salama MM, Fathy MM, Soliman FM, R. Saber F. Cattleianal and Cattleianone: Two New Meroterpenoids from Psidium cattleianum Leaves and Their Selective Antiproliferative Action against Human Carcinoma Cells. Molecules. 2021; 26(10):2891. https://doi.org/10.3390/molecules26102891
Chicago/Turabian StyleA. Mahrous, Engy, Ahmed M. Al-Abd, Maha M. Salama, Magda M. Fathy, Fathy M. Soliman, and Fatema R. Saber. 2021. "Cattleianal and Cattleianone: Two New Meroterpenoids from Psidium cattleianum Leaves and Their Selective Antiproliferative Action against Human Carcinoma Cells" Molecules 26, no. 10: 2891. https://doi.org/10.3390/molecules26102891
APA StyleA. Mahrous, E., Al-Abd, A. M., Salama, M. M., Fathy, M. M., Soliman, F. M., & R. Saber, F. (2021). Cattleianal and Cattleianone: Two New Meroterpenoids from Psidium cattleianum Leaves and Their Selective Antiproliferative Action against Human Carcinoma Cells. Molecules, 26(10), 2891. https://doi.org/10.3390/molecules26102891