The Physicochemical Characteristics of Gelam Honey and Its Outcome on the Female Reproductive Tissue of Sprague–Dawley Rats: A Preliminary Study
Abstract
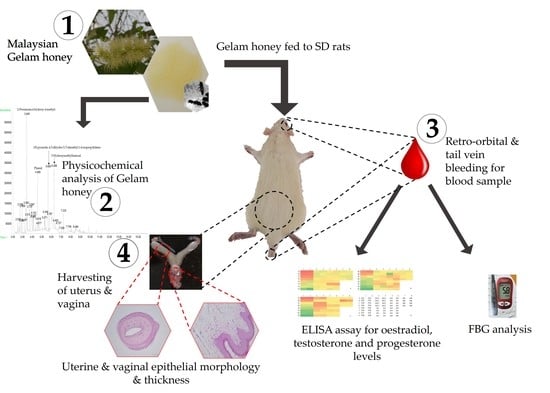
:1. Introduction
2. Results
2.1. Physicochemical Profile of Gelam Honey for Quality Determination
2.2. Body Weight, Food Intake and FBG and Serum Hormonal Profile of Gelam Honey-Treated Rats
2.3. Uterine and Vaginal Epithelial Thickness Measurement
3. Discussion
4. Materials and Methods
4.1. Honey Sample
4.2. Physicochemical Profiling of Gelam Honey for Quality Determination
4.2.1. Determination of Hydroxymethylfurfural (HMF)
4.2.2. Moisture Content
4.2.3. Sugar Profiling
4.2.4. Semi-Volatile Organic Compound (SVOC) Determination
4.3. Ethical Approval of Experimental Animals
4.4. Animal Care and Handling
4.5. Experimental Design
4.6. Fasting Blood Glucose (FBG) Measurement
4.7. Serum Hormone Profile
4.8. Uterine and Vaginal Epithelial Thickness Measurement
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complementary Altern. Med. 2013, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2723–S2738. [Google Scholar] [CrossRef]
- Crane, E. Chapter 9-Apis Species: (Honey Bees). In Encyclopedia of Insects; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Berkeley, CA, USA, 2009; pp. 31–32. [Google Scholar] [CrossRef]
- Azmi, M.F.; Abd Ghafar, N.; Che Hamzah, J.; Chua, K.H.; Ng, S.L. The role of Gelam honey in accelerating reepithelialization of ex vivo corneal abrasion model. J. Food Biochem. 2021, e13645. [Google Scholar] [CrossRef]
- Malaysian Standard. MS 2683: 2017: Kelulut (Stingless Bee) Honey-Specification: Quality Requirements; International Honey Commission: Selangor, Malaysia, 2017. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products-Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 43–82. [Google Scholar] [CrossRef]
- Crane, E. Chapter 20-Bee products. In Encyclopedia of Insects; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Berkeley, CA, USA, 2009; pp. 71–75. [Google Scholar] [CrossRef]
- Ball, D.W. The chemical composition of honey. J. Chem. Educ. 2007, 84, 1643. [Google Scholar] [CrossRef]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Mijanur Rahman, M.; Gan, S.H.; Khalil, M. Neurological effects of honey: Current and future prospects. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ibrahim, S.F.; Jaffar, F.H.F.; Mokhtar, M.H.; Chin, K.Y.; Osman, K. Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules 2021, 26, 649. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef]
- Khalil, M.L.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart disease: A review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7. Available online: https://www.ajol.info/index.php/ajtcam/article/view/56693 (accessed on 15 March 2021). [CrossRef] [PubMed] [Green Version]
- Yao, L.K.; Razak, S.L.; Ismail, N.; Fai, N.C.; Asgar, M.H.; Sharif, N.M.; Aan, G.J.; Jubri, Z. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J. Med. Plant Res. 2011, 5, 5618–5625. [Google Scholar]
- Ibeanu, O.; Modesitt, S.C.; Ducie, J.; Von Gruenigen, V.; Agueh, M.; Fader, A.N. Hormone replacement therapy in gynecologic cancer survivors: Why not? Gynecol. Oncol. 2011, 122, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.G., Jr.; Rosas, F.C.; Oneda, B.; Labes, E.; Tinucci, T.; Abrahão, S.B.; da Fonseca, A.M.; Mion, D., Jr.; de Moraes Forjaz, C.L. Aerobic training abolishes ambulatory blood pressure increase induced by estrogen therapy: A double blind randomized clinical trial. Maturitas 2011, 69, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Venier, M. The controversial history of hormone replacement therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius. International Standard for Honey CXS 12-19811 Adopted in 1981; Amended in 2019; FAO: Rome, Italy, 1981; pp. 1–8. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.M.; Gurtu, S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. In Annales D’endocrinologie; Elsevier: Masson, IA, USA, 2010; Volume 71, pp. 291–296. [Google Scholar] [CrossRef]
- NCBI (National Center for Biotechnology Information). PubChem Compound Summary for CID 31256, Diacetone Alcohol. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diacetone-alcohol (accessed on 16 March 2021).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. Metabocard for Diacetone alcohol (HMDB0031511). HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D17–D608. [Google Scholar] [CrossRef]
- Wånggren, K.; Stavreus-Evers, A.; Olsson, C.; Andersson, E.; Gemzell-Danielsson, K. Regulation of muscular contractions in the human Fallopian tube through prostaglandins and progestagens. Hum. Reprod. 2008, 23, 2359–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Igwaran, A.; Iweriebor, B.C.; Okoh, S.O.; Nwodo, U.U.; Obi, L.C.; Okoh, A.I. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community Eastern Cape, South Africa. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Zottele, I.; Piccoli, P.; Tamanini, A.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanomaterial gas sensors for online monitoring system of fruit jams. Foods 2019, 8, 632. [Google Scholar] [CrossRef] [Green Version]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Takei, Y.; Kusakabe, M.; Sakamoto, T. Hormonal regulation of thirst in the amphibious ray-finned fish suggests the requirement for terrestrialization during evolution. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, A.N.; Hsiao, S. Angiotensin as Dipsogen. In Control Mechanisms of Drinking; Peters, G., Fitzsimons, J.T., Peters-Haefeli, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar] [CrossRef]
- Ali, A.M.; Hendawy, A.O. Bee honey as a potentially effective treatment for depression: A review of clinical and preclinical findings. JOJ Nurs. Health Care 2018, 9, 555764. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and melissopalynological characterization of Estonian summer honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey—A Novel Antidiabetic Agent. Int. J. Biol. Sci. 2012, 8, 913–934. [Google Scholar] [CrossRef] [Green Version]
- Ramli, E.S.M.; Sukalingam, K.; Kamaruzzaman, M.A.; Soelaiman, I.N.; Pang, K.L.; Chin, K.Y. Direct and Indirect Effect of Honey as a Functional Food Against Metabolic Syndrome and Its Skeletal Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 241. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [Green Version]
- Abraham, G.E. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. Endocrinol. Metab. 1974, 39, 340–346. [Google Scholar] [CrossRef]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77, 3–5. [Google Scholar] [CrossRef]
- Davison, S.L.; Bell, R.; Donath, S.; Montalto, J.G.; Davis, S.R. Androgen levels in adult females: Changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005, 90, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The genitourinary syndrome of menopause: An overview of the recent data. Cureus 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- White, J.W., Jr. Spectrophotometric method for hydroxymethylfurfural in honey. J. AOAC Int. 1979, 62, 509–514. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Huang, B.; Hu, J.; Rohrer, J. Determination of carbohydrates in kombucha using HPAE-PAD. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2016; Volume 252. [Google Scholar]
- U.S. EPA. Method 8270E (SW-846): Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); U.S. EPA: Washington, DC, USA, 2014. Available online: https://www.epa.gov/esam/epa-method-8270e-sw-846-semivolatile-organic-compounds-gas-chromatographymass-spectrometry-gc (accessed on 16 March 2021).
- Moniruzzaman, M.; Rodríguez, I.; Ramil, M.; Cela, R.; Sulaiman, S.A.; Gan, S.H. Assessment of gas chromatography time-of-flight accurate mass spectrometry for identification of volatile and semi-volatile compounds in honey. Talanta 2014, 129, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Suratman, M.N.; Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, Selangor, Malaysia. Personal communication, 2021.














| HMF (mg/100 g) | Moisture Content (%) | |
|---|---|---|
| Gelam honey | 6.9 | 18.75 |
| Tualang honey | 7.86 [11] | 20 [19] |
| CODEX CXS 12-1981 | <8 | <21 |
| Glucose (g/100 g) | Fructose (g/100 g) | Sucrose (g/100 g) | Maltose (g/100 g) | Reducing Sugars (%) | Total Fructose and Glucose (g/100 g) | Fructose/Glucose Ratio | Glucose/Water Ratio | |
|---|---|---|---|---|---|---|---|---|
| GH * | 40.05 | 35.35 | undetected | 1.99 | 77.5 | 75.40 | 0.88 | 2.14 |
| TH * [18] | 30.0 | 29.6 | 0.6 | 7.9 | 67.5 | 59.6 | 0.99 | 1.5 |
| MS 2683:2017 | NS | NS | <7.5 | NS | NS | <85 | 0.9–1.35 | NS |
| CODEX CXS 12-1981 | NS | NS | <5 | NS | >65 | ≥60 | NS | NS |
| RT (min) | Percentage of Total (%) | Cas. No. | MW (Da) | ||
|---|---|---|---|---|---|
| Benzene derivatives | |||||
| Benzene, (1-methylethyl)- | 4.141 | 2.12 | 98-82-8 | 120.1916 | Aromatic, Common name: cumene |
| Alcohols | |||||
| 2-Furanmethanol | 3.513 | 18.62 | 98-00-0 | 98.0999 | |
| Alkenes, alkanes and alkynes | |||||
| α-Methylstyrene | 4.618 | 9.08 | 98-83-9 | 118.1757 | Olefinic compound |
| Non-aromatic ketones and aldehydes | |||||
| 2-Pentanone, 4-hydroxy-4-methyl | 3.440 | 4.40 | 123-42-2 | 116.1583 | Common name: diacetone alcohol. Beta-hydroxy ketone |
| 1,3-Dihydroxyacetone dimer | 3.947 | 3.85 | 26776-70-5 | 180.16 | |
| 5-Hydroxymethylfurfural | 6.269 | 10.04 | 67-47-0 | 126.1100 | |
| Aromatic ketones and aldehydes | |||||
| 3-Penten-2-one, 4-methyl | 2.9258 | 3.94 | 141-79-7 | 98.143 | Olefinic compound |
| 2-Cyclopenten-1-one, 2-hydroxy- | 4.127 | 2.31 | 10493-98-8 | 98.0999 | |
| Phenols and its derivatives | |||||
| Phenol | 4.565 | 2.55 | 108-95-2 | 94.1112 | Carbolic acid, aromatic compound |
| 4H-Pyran-4-one, 2,3-Dihydro-3,5-dihydroxy-6-methyl- | 5.787 | 2.67 | 28564-83-2 | 144.1253 | Flavonoid |
| Furans and furanone | |||||
| Furyl hydroxymethyl ketone | 5.349 | 3.04 | 17678-19-2 | 126.1100 | |
| Other hydrocarbons | |||||
| 1H-Pyrazole 4 5-dihydro-5 5-Dimethyl-4-isopropylidene- | 5.655 | 9.09 | 28019-94-5 | 98.1463 |
| Group | Initial Body Weight (g) | Final Body Weight (g) | Body Weight Change (%) | Food Intake (g/Day) | Water Intake (mL/Day) |
|---|---|---|---|---|---|
| GH0.2 | 200 ± 6.976 a | 218.5 ± 5.752 | +8.28 | 12.04 b | 33.39 c |
| GH1 | 240.75 ± 12.058 a | 243.75 ± 14.442 | +0.86 | 17.80 b | 23.75 c |
| GH2 | 212.50 ± 7.399 | 215 ± 5.212 | +1.21 | 16.19 b | 21.7 c |
| GH8 | 235.25 ± 8.826 | 221.25 ± 5.618 | ‒6.37 | 17.11 b | 21.43 c |
| Group | Testosterone (ng/mL) | Progesterone (ng/mL) | Estradiol (pg/mL) |
|---|---|---|---|
| GH0.2 | 7.05 ± 0.66 | 10.92 ± 0.4 | 16.25 ± 1.27 |
| GH1 | 8.55 ± 0.62 | 9.63 ± 0.98 | 18.40 ± 4.68 |
| GH2 | 8.75 ± 0.87 | 9.44 ± 0.5 | 19.95 ± 4.31 |
| GH8 | 7.04 ± 0.38 | 9.53 ± 0.66 | 15.9 ± 2.26 |
| Reference range for healthy normal female rats * | 4.97–6.58 | 4.1–8.2 | 67–99 |
| Groups | Uterine Epithelial Thickness (µm) | Vagina Epithelial Thickness (µm) |
|---|---|---|
| GH0.2 | 9.84 ± 0.16 | 47.56 ± 0.88 |
| GH1 | 21.74 ± 0.31 | 43.09 ± 0.93 |
| GH2 | 23.06 ± 0.38 | 75.17 ± 1.91 |
| GH8 | 22.84 ± 0.19 | 42.43 ± 0.59 |
| Physicochemical Parameters | GH |
|---|---|
| Color | amber |
| Hydroxymethylfurfural (mg/100 g) | 6.9 |
| Moisture content (%) | 18.75 |
| Glucose (g/100 g) | 40.05 |
| Fructose (g/100 g) | 35.35 |
| Sucrose (g/100 g) | undetected |
| Maltose (g/100 g) | 1.99 |
| Reducing sugars (%) | 77.5 |
| Sum of fructose and glucose (g/100 g) | 75.4 |
| Fructose/glucose ratio | 0.88 |
| Glucose/water ratio | 2.14 |
| Column | Thermo Scientific Dionex CarboPac PA20 Analytical Column (3 × 150 mm) |
|---|---|
| Eluent source | Merck NaOH solution 50% |
| Eluent | A—200 mM NaOH, B—ultrapure water |
| Isocratic | 5–0 min: 9 mM NaOH (equilibration) 0–24 min: 9 mM NaOH 24–24.1 min: 9–200 mM NaOH 24.1–29 min: 200 mM NaOH 29–29.1 min: 200–9 mM NaOH 29.1–40 min: 9 mM NaOH |
| Flow rate | 0.5 mL/min |
| Injection volume | 25 μL |
| Temperature | 30 °C (column and detector compartments) |
| Backpressure | 2500–3000 psi |
| Detection | Electrochemical detector |
| Background | 6–10 nC |
| Working electrode | Gold electrode |
| Electrochemical cell gasket | Ultem™ 15 mil (1 mil = 0.001 inches) |
| Reference electrode | pH, Ag/AgCl, Ag mode |
| Noise | - |
| GC Parameters | |
|---|---|
| Inlet mode | Split-less |
| Split-less time (min) | 16 |
| Carrier gas, flow, flow rate | Helium, constant pressure 10 psi, 1.7 mL/min |
| Oven | 50 °C, 0.1 min |
| Chromatographic column | 30 m × 0.25 mm internal diameter × 0.5 μm film thickness DB-UI-8270D ULTRA INERT (Agilent Technologies, Santa Clara, CA, USA) |
| MS Parameters | |
| Transfer line temperature (°C) | 300 |
| Source temperature (°C) | 230 |
| Ionization mode | Electron ionization (EI) |
| Electron energy (eV) | 70 |
| Acquisition mode | Full scan 40–650 m/z |
| MS library | NIST MS Search 2.2 (Gaithersburg, MD, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, N.H.; Osman, K.; Zulkefli, A.F.; Mokhtar, M.H.; Ibrahim, S.F. The Physicochemical Characteristics of Gelam Honey and Its Outcome on the Female Reproductive Tissue of Sprague–Dawley Rats: A Preliminary Study. Molecules 2021, 26, 3346. https://doi.org/10.3390/molecules26113346
Ismail NH, Osman K, Zulkefli AF, Mokhtar MH, Ibrahim SF. The Physicochemical Characteristics of Gelam Honey and Its Outcome on the Female Reproductive Tissue of Sprague–Dawley Rats: A Preliminary Study. Molecules. 2021; 26(11):3346. https://doi.org/10.3390/molecules26113346
Chicago/Turabian StyleIsmail, Nur Hilwani, Khairul Osman, Aini Farzana Zulkefli, Mohd Helmy Mokhtar, and Siti Fatimah Ibrahim. 2021. "The Physicochemical Characteristics of Gelam Honey and Its Outcome on the Female Reproductive Tissue of Sprague–Dawley Rats: A Preliminary Study" Molecules 26, no. 11: 3346. https://doi.org/10.3390/molecules26113346
APA StyleIsmail, N. H., Osman, K., Zulkefli, A. F., Mokhtar, M. H., & Ibrahim, S. F. (2021). The Physicochemical Characteristics of Gelam Honey and Its Outcome on the Female Reproductive Tissue of Sprague–Dawley Rats: A Preliminary Study. Molecules, 26(11), 3346. https://doi.org/10.3390/molecules26113346