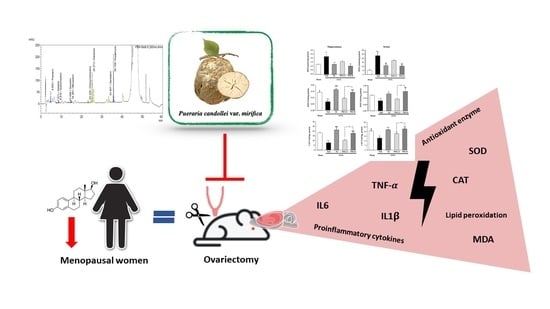
Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain
Abstract
:1. Introduction
2. Results
2.1. Effects of PM Extract and Estrogen on OVX-Induced Cognitive Impairments
2.2. Changes in Uterus Weight and Volume and Serum 17β-Estradiol Levels after the PM Treatment
2.3. Effects of PM Extract and Estrogen on Oxidative Damage and Antioxidant Enzyme Activities in the Hippocampus and Serum of OVX Mice
2.4. Effects of PM Extract and Estrogen on Proinflammatory Cytokines and Estrogen-Mediated Gene in Hippocampus
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extraction
4.2. Animals
4.3. Surgical Procedure
4.4. Behavioral Test
4.4.1. Y-Maze Test
4.4.2. Novel Object Recognition Test (NORT)
4.4.3. Morris Water Maze (MWM) Test
4.5. Measurement of Serum 17β-Estradiol Levels
4.6. Dissection of Uterus and Brain Tissues
4.7. Oxidative Stress Measurement
4.7.1. Lipid Oxidative Damage
4.7.2. Assessment of Enzymatic Antioxidant Defenses
4.8. Quantitative Real-Time PCR (QPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasbarri, A.; Tavares, M.C.H.; Rodrigues, R.C.; Tomaz, C.; Pompili, A. Estrogen, cognitive functions and emotion: An overview on humans, non-human primates and rodents in reproductive years. Rev. Neurosci. 2012, 23, 587–606. [Google Scholar] [CrossRef]
- Luine, V.; Frankfurt, M. Estrogenic regulation of memory: The first 50 years. Horm. Behav. 2020, 121, 104711. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neuro-degenerative disease. J. Neuroinflamm. 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Li, S.R.; Li, X.W.; Chen, X.; Wei, Z.X.; Liu, Q.S.; Cheng, Y. The effect of estrogen replacement therapy on Alz-heimer’s disease and Parkinson’s disease in postmenopausal women: A meta-analysis. Front. Neurosci. 2020, 14, 157. [Google Scholar] [CrossRef]
- Volicer, L. Physiological and pathological functions of beta-amyloid in the brain and alzheimer’s disease: A review. Chin. J. Physiol. 2020, 63, 95. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Milne, M.R.; Waldvogel, H.J.; Faull, R.L. Effect of estradiol on neurotrophin receptors in basal forebrain cholinergic neurons: Relevance for Alzheimer’s disease. Int. J. Mol. Sci. 2016, 17, 2122. [Google Scholar] [CrossRef] [Green Version]
- Kishida, K.T.; Klann, E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. ARS 2007, 9, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Monthakantirat, O.; Sukano, W.; Umehara, K.; Noguchi, H.; Chulikhit, Y.; Matsumoto, K. Effect of miroestrol on ovari-ectomy-induced cognitive impairment and lipid peroxidation in mouse brain. Phytomedicine 2014, 21, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Murali, G.; Panneerselvam, C. Age-related oxidative protein damages in central nervous system of rats: Modulatory role of grape seed extract. Int. J. Dev. Neurosci. 2005, 23, 501–507. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, estrogen and female brain aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Cagnacci, A.; Venier, M. The controversial history of hormone replacement therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [Green Version]
- Khamphukdee, C.; Monthakantirat, O.; Chulikhit, Y.; Buttachon, S.; Lee, M.; Silva, A.; Sekeroglu, N.; Kijjoa, A. Chemical constituents and antidepressant-like effects in ovariectomized mice of the ethanol extract of Alternanthera philoxeroides. Molecules 2018, 23, 2202. [Google Scholar] [CrossRef] [Green Version]
- Suntara, A. The Remedy Pamphlet of Kwao Krua Tuber of Luang Anusarnsuntarakromkarnphiset; Chiang Mai Upatipongsa Press: Chiang Mai, Thailand, 1931. [Google Scholar]
- Tantipongpiradet, A.; Monthakantirat, O.; Vipatpakpaiboon, O.; Khampukdee, C.; Umehara, K.; Noguchi, H.; Fujiwara, H.; Matsumoto, K.; Sekeroglu, N.; Kijjoa, A.; et al. Effects of puerarin on the ovariectomy-induced depressive-like behavior in ICR mice and its possible mechanism of action. Molecules 2019, 24, 4569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiriyakarun, S.; Yodpetch, W.; Komatsu, K.; Zhu, S.; Ruangrungsi, N.; Sukrong, S. Discrimination of the Thai rejuvenating herbs Pueraria candollei (White Kwao Khruea), Butea superba (Red Kwao Khruea), and Mucuna collettii (Black Kwao Khruea) using PCR-RFLP. J. Nat. Med. 2013, 67, 562–570. [Google Scholar] [CrossRef]
- Malaivijitnond, S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front. Med. 2012, 6, 8–21. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Udomsuk, L.; Monthakantirat, O.; Churikhit, Y.; Putalun, W.; Jarukamjorn, K. Effects of Pueraria mirifica and miroestrol on the antioxidation-related enzymes in ovariectomized mice. J. Pharm. Pharmacol. 2013, 65, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Chansakaow, S.; Ishikawa, T.; Sekine, K.; Okada, M.; Higuchi, Y.; Kudo, M.; Chaichantipyuth, C. Isoflavonoids from Pueraria mirifica and their estrogenic activity. Planta Med. 2000, 66, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Manonai, J.; Chittacharoen, A.; Udomsubpayakul, U.; Theppisai, H.; Theppisai, U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause 2008, 15, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Udomsuk, L.; Chatuphonprasert, W.; Monthakantirat, O.; Churikhit, Y.; Jarukamjorn, K. Impact of Pueraria candollei var. mirifica and its potent phytoestrogen miroestrol on expression of bone-specific genes in ovariectomized mice. Fitoterapia 2012, 83, 1687–1692. [Google Scholar] [CrossRef]
- Chandeying, V.; Lamlertkittikul, S. Challenges in the conduct of Thai herbal scientific study: Efficacy and safety of phy-toestrogen, Pueraria mirifica (Kwao Keur Kao), phase I, in the alleviation of climacteric symptoms in perimenopausal women. J. Med. Assoc. Thai. 2007, 90, 1274. [Google Scholar] [PubMed]
- Anukulthanakorn, K.; Parhar, I.S.; Jaroenporn, S.; Kitahashi, T.; Watanbe, G.; Malaivijitnond, S. Neurotherapeutic effects of Pueraria mirifica extract in early-and late-stage cognitive impaired rats. Phytother. Res. 2016, 30, 929–939. [Google Scholar] [CrossRef]
- Smiley, D.A.; Khalil, R.A. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr. Med. Chem. 2009, 16, 1863–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udomsin, O.; Juengwatanatrakul, T.; Yusakul, G.; Putalun, W. Chromene stability: The most potent estrogenic compounds in white Kwao Krua (Pueraria candollei var mirifica) crude extract. J. Funct. Foods 2015, 19, 269–277. [Google Scholar] [CrossRef]
- Kraithong, W.; Juengsanguanpornsuk, W.; Krittanai, S.; Yusakul, G.; Putalun, W. Comparative analysis of the chemical constituents from the tuberous root and stem of Pueraria candollei var. mirifica and evaluation of their estrogenic activity. Pharmacogn. Mag. 2020, 16, 353. [Google Scholar] [CrossRef]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef]
- Berry, A.; Greco, A.; Giorgio, M.; Pelicci, P.G.; De Kloet, R.; Alleva, E.; Minghetti, L.; Cirulli, F. Deletion of the lifespan determinant p66Shc improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult mice. Exp. Gerontol. 2008, 43, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.M.F.; Banin, R.M.; Thomaz, F.M.; de Andrade, I.S.; Boldarine, V.T.; de Souza Figueiredo, J.; Hirata, B.K.S.; Oyama, L.M.; Lago, J.H.G.; Ribeiro, E.B.; et al. Ginkgo biloba extract (GbE) restores serotonin and leptin receptor levels and plays an antioxidative role in the hippocampus of ovariectomized rats. Mol. Neurobiol. 2021, 58, 2693–2707. [Google Scholar] [CrossRef] [PubMed]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019, 55, 100787. [Google Scholar] [CrossRef]
- Cherdshewasart, W.; Subtang, S.; Dahlan, W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J. Pharm. Biomed. Anal. 2007, 43, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.; Pieri, F.; Fung, B.; Dani, J.A.; Biasi, M.D. Altered anxiety-related responses in mutant mice lacking the β4 Subunit of the Nicotinic Receptor. J. Neurosci. 2003, 23, 6255–6263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y.; Kijjoa, A. Kleeb Bua Daeng, a Thai traditional herbal formula, ameliorated unpredictable chornic mild stress-induced cognitive impairment in ICR mice. Molecules 2019, 24, 4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Treatment | Uterus | Serum E2 (pg/mL) | |
|---|---|---|---|
| Weight (g) | Volume (cm3) | ||
| Sham | 0.227 ± 0.014 | 0.153 ± 0.022 | 24.63 ± 2.27 |
| OVX + vehicle | 0.069 ± 0.008 * | 0.054 ± 0.009 * | 13.03 ± 2.38 * |
| OVX + E2 (1 μg/kg/day) | 0.277 ± 0.028 # | 0.135 ± 0.010 # | 32.88 ± 4.99 ## |
| OVX + PM (2.5 mg/kg/day) | 0.199 ± 0.009 # | 0.163 ± 0.006 # | 16.10 ± 2.51 |
| OVX + PM (25 mg/kg/day) | 0.226 ± 0.013 # | 0.179 ± 0.016 # | 23.74 ± 2.95 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulikhit, Y.; Sukhano, W.; Daodee, S.; Putalun, W.; Wongpradit, R.; Khamphukdee, C.; Umehara, K.; Noguchi, H.; Matsumoto, K.; Monthakantirat, O. Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain. Molecules 2021, 26, 3442. https://doi.org/10.3390/molecules26113442
Chulikhit Y, Sukhano W, Daodee S, Putalun W, Wongpradit R, Khamphukdee C, Umehara K, Noguchi H, Matsumoto K, Monthakantirat O. Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain. Molecules. 2021; 26(11):3442. https://doi.org/10.3390/molecules26113442
Chicago/Turabian StyleChulikhit, Yaowared, Wichitsak Sukhano, Supawadee Daodee, Waraporn Putalun, Rakvajee Wongpradit, Charinya Khamphukdee, Kaoru Umehara, Hiroshi Noguchi, Kinzo Matsumoto, and Orawan Monthakantirat. 2021. "Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain" Molecules 26, no. 11: 3442. https://doi.org/10.3390/molecules26113442
APA StyleChulikhit, Y., Sukhano, W., Daodee, S., Putalun, W., Wongpradit, R., Khamphukdee, C., Umehara, K., Noguchi, H., Matsumoto, K., & Monthakantirat, O. (2021). Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain. Molecules, 26(11), 3442. https://doi.org/10.3390/molecules26113442