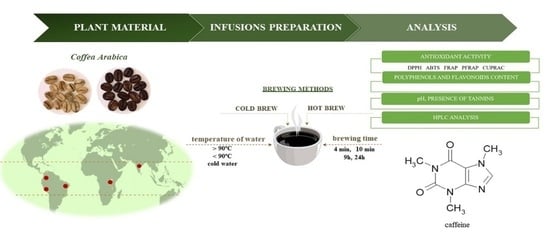
The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Antioxidant Activity of Coffee Infusion
2.2. Total Polyphenols and Flavonoids Content
2.3. Caffeine Content in Coffee Infusion
2.4. Tannins Content and pH of Coffee Infusions
2.5. Statistical Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Coffee Brews
3.3. Evaluation of Antioxidant Activity
3.4. Evaluation of Total Polyphenols and Flavonoids Content
3.5. Evaluation of Tannins Content and pH of Infusions
3.6. HPLC Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, A.; Marques, L.; Gonçalves, C.; Marcucci, M. Botanical aspects, caffeine content and antioxidant activity of Coffea arabica. Am. J. Plant. Sci. 2019, 10, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Goodin, M.; Dos Reis Figueira, A. Good to the last drop: The emergence of coffee ringspot virus. PLoS Pathog. 2019, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Matysek-Nawrocka, M.; Cyrankiewicz, P. Biological active substances derived from tea, coffee and cocoa and their application in cosmetics. Post. Fitoter. 2016, 17, 139–144. [Google Scholar]
- Krishnan, S. Sustainable coffee production. ORE Environ. Sci. 2017, 1–34. [Google Scholar] [CrossRef]
- Kohlmünzer, S. Pharmacognosy, 5th ed.; Wyd. Lek. PZWL: Warsaw, Poland, 2013; pp. 475–476. [Google Scholar]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj J. 2021, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rebacz-Maron, E.; Gutowska, I. Mineral composition and antioxidant potential of coffee beverages depending on the brewing method. Foods 2020, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, B.S. A colorimetric sensor array-based classification of coffees. Sens. Actuators B Chem. 2018, 275, 277–283. [Google Scholar] [CrossRef]
- Gonzales, E.C.I.; Lloren, K.G.M.; Al-shdifat, J.S.; Valdez, L.B.; Gines, K.R.; Garcia, E.V. Effect of pressure on the particle size distribution of espresso coffee. KIMIKA 2018, 29, 30–35. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Leonardo Moreno, F.; Yolanda Ruiz, R. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2018, 99, 391–399. [Google Scholar] [CrossRef]
- Amanpour, A.; Selli, S. Differentiation of volatile profiles and odor activity values of Turkish coffee and French press coffee. J. Food Process. Preserv. 2016, 40, 1116–1124. [Google Scholar] [CrossRef]
- Mubarak, A.; Croft, K.D.; Bondonno, C.B.; Din, N.S. Comparison of liberica and arabica coffee: Chlorogenic acid, caffeine, total phenolic and DPPH radical scavenging activity. Asian J. Agric. Biol. 2019, 7, 130–136. [Google Scholar]
- Affonso, R.; Voytena, A.; Fanan, S.; Pitz, H.; Coelho, D.; Horstmann, A.; Pereira, A.; Uarrota, V.; Hellmann, M.; Ravela, L.; et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of Coffee (Coffea arabica L.) Bean residual press cake on the skin wound healing. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. BioMed. Res. Int. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Bogdański, P. Health-promoting properties of coffee. Varia Med. 2019, 3, 311–317. [Google Scholar]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee by products in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Witkowska, A.; Mirończuk-Chodakowska, I.; Terlikowska, K.; Kulesza, K.; Zujko, M. Coffee and its biologically active components: Is there a connection to breast, endometrial, and ovarian cancer?—A review. Pol. J. Food Nutr. Sci. 2020, 70, 207–222. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Priftis, A.; Stagos, D.; Konstantinopoulos, K.; Tsitsimpikou, C.; Spandidos, D.A.; Tsatsakis, A.M.; Tzatzarakis, M.N.; Kouretas, D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 2015, 12, 7293–7302. [Google Scholar] [CrossRef] [PubMed]
- Dybkowska, E.; Sadowska, A.; Rakowska, R.; Dębowska, M.; Świderski, F.; Świąder, K. Assessing polyphenols content and antioxidant activity in coffee beans according to origin and the degree of roasting. Rocz. Panstw. Zakl. Hig. 2017, 68, 347–353. [Google Scholar] [PubMed]
- Ribeiro, E.; de Souza Rocha, T.; Prudencio, S.H. Potential of green and roasted coffee beans and spent coffee grounds to provide bioactive peptides. Food Chem. 2021, 348, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2020, 246, 437–448. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical characteristics of hot and cold brew coffee chemistry: The effects of roast level and brewing temperature on compound extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Castiglioni, S.; Damiani, E.; Astolfi, P.; Carloni, P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. Int. J. Food Sci. Nutr. 2015, 66, 491–497. [Google Scholar] [CrossRef]
- Pramudya, R.C.; Seo, H.S. Influences of product temperature on emotional responses to, and sensory attributes of, coffee and green tea beverages. Front. Psychol. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Górecki, M.; Hallmann, E. The antioxidant content of coffee and its in vitro activity as an effect of its production method and roasting and brewing time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Fibrianto, K.; Umam, K.; Wulandari, E.S. Effect of roasting profiles and brewing methods on the characteristics of bali kintamani coffee. Adv. Eng. Res. 2018, 172, 194–197. [Google Scholar]
- Merecz, A.; Marusińska, A.; Karwowski, B.T. The content of biologically active substances and antioxidant activity in coffee depending on brewing method. Pol. J. Natur. Sci. 2018, 33, 267–284. [Google Scholar]
- Motora, K.G.; Beyene, T.T. Determination of caffeine in raw and roasted coffee beans of ilu abba bora zone, South West Ethiopia. Indo Am. J. Pharm. Res. 2017, 7, 463–470. [Google Scholar]
- Gebeyehu, B.T.; Bikila, S.L. Determination of caffeine content and antioxidant activity of coffee. Am. J. Appl. Chem. 2015, 3, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cόrdoba, N.; Moreno, F.L.; Coralia, O.; Velaśqueaz, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: The influence of espresso machines and coffee cultivars. Int. J. Food Sci. Nutr. 2014, 65, 465–469. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int. J. Food Sci. Nutr. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Zaguła, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmiański, J. Comparison of the effectiveness of water-based extraction of substances from dry tea leaves with the use of magnetic field assisted extraction techniques. Molecules 2017, 22, 1656. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.; Koh, E. Spent coffee as a rich source of antioxidative compounds. Food Sci. Biotechnol. 2017, 26, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Patay, É.B.; Sali, N.; Kőszegi, T.; Csepregi, R.; Balázs, V.L.; Németh, T.S.; Németh, T.; Papp, N. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three Coffea species. Asian Pac. J. Trop Med. 2016, 9, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Andueza, S.; Vila, M.A.; Paz de Peña, M.; Cid, C. Influence of coffee/water ratio on the final quality of espresso coffee. J. Sci. Food Agric. 2007, 87, 586–592. [Google Scholar] [CrossRef]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Klimowicz, A. The antioxidant potential of flesh, albedo and flavedo extracts from different varieties of grapefruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 453–462. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]




| Area of Impact/Type of Dysfunction | Effect |
|---|---|
| The nervous system The central nervous system (CNS) - Alzheimer’s disease - Parkinson’s disease - Depression Peripheral nervous system - Sense organs | An adenosine receptor antagonist—caffeine stimulates the CNS, improves concentration and thought processes [16,17,18,19]. Coffee consumption improves the metabolism and uptake of the antiparkinsonian drugs, which reduces the latency to a motor response and reduces the risk of Parkinson’s disease [16,17,18]. Caffeine stimulates the secretion of serotonin and dopamine and, in the proper dose, it can reduce the risk of depression [17]. The soluble and volatile infusion components provide aroma and flavor [17,18]. |
| The gastrointestinal system | Caffeine stimulates gastric acid secretion by activating bitter taste receptors, what decreases the pH of the gastric juice. Low pH improves the solubility of weakly alkaline drugs as they form soluble salts [16]. |
| Obesity and type II diabetes (metabolic disorders) | Chlorogenic acid and trigonelline significantly reduce blood glucose and insulin levels [17,18,19]. Cafestol increases insulin sensitivity in muscle cells [17,18,19]. Chlorogenic acid intensifies the adipose tissue decomposition, and caffeine inhibits the absorption of fatty acids in the intestinal lumen [17,18,19]. Infusions are characterized by a low calorific value [17]. |
| The cardiovascular system | Antioxidants improve the bioavailability of nitric oxide in the vascular system and enhance the vascular endothelium [17,18,19]. |
| Carcinogenesis | Components contained in coffee repair and protect DNA against oxidative damage, show anti-inflammatory activity, increase apoptosis of cancer cells, and cause tumor suppression [17,18,19,20]. |
| Large intestine | Coffee increases intestinal peristalsis, excretion of bile acids with the feces, and modifies the composition of the intestinal microbiota [19]. |
| Breast cancer | Phytohormones in coffee increase the concentration of globulin, which binds sex hormones and reduces the absorption of testosterone and affects the level of luteal estrogens [17,20]. |
| Ovarian cancer | Chlorogenic, caffeic acids, and caffeine show antiproliferative properties in ovarian cancer cells. Cytochrome P450 isozymes involved in caffeine metabolism may contribute to the development of ovarian cancer or reduce the risk of its occurrence [17,19]. |
| Endometrial cancer (EC) | This effect of coffee is associated with the correlation between the prevalence of obesity and EC and with the increased sensitivity of cellular receptors to insulin [19]. |
| Signature | Description of the Brewing Methods |
|---|---|
| Cold brew | |
| CB, 9 h | The ground grains were poured over with boiled and cooled tap water at 23–25 °C and stored at +4 °C for 9 h. |
| CB, 24 h | The ground grains were poured over with boiled and cooled tap water at 23–25 °C and stored at +4 °C for 24 h. |
| Hot brew | |
| 86 °C, 4’ | The ground grains were poured over with boiled tap water at 84–86 °C and brewing for 4 min. |
| 86 °C, 10’ | The ground grains were poured over with boiled tap water at 84–86 °C and brewing for 10 min. |
| 95 °C, 4’ | The ground grains were poured over with boiled tap water at 93–95 °C and brewing for 4 min. |
| 95 °C, 10’ | The ground grains were poured over with boiled tap water at 93–95 °C and brewing for 10 min. |
| Brewing Method | mg/100 mL of Coffee Infusion | |
|---|---|---|
| Unroasted Bean Infusion | Roasted Bean Infusion | |
| Brazil | ||
| CB, 9 h | 54.13 ± 1.35 | 48.87 ± 0.08 |
| CB, 24 h | 51.18 ± 0.59 | 67.72 ± 2.36 |
| 86 °C, 4’ | 34.50 ± 0.52 | 46.00 ± 0.11 |
| 86 °C, 10’ | 42.68 ± 0.33 | 47.53 ± 0.18 |
| 95 °C, 4’ | 43.22 ± 0.46 | 53.07 ± 0.39 |
| 95 °C, 10’ | 29.90 ± 0.32 | 49.08 ± 0.63 |
| Colombia | ||
| CB, 9 h | 62.30 ± 0.55 | 50.61 ± 0.17 |
| CB, 24 h | 60.11 ± 0.17 | 57.04 ± 0.82 |
| 86 °C, 4’ | 36.02 ± 0.03 | 52.78 ± 0.57 |
| 86 °C, 10’ | 37.64 ± 0.20 | 48.65 ± 0.24 |
| 95 °C, 4’ | 34.26 ± 0.12 | 56.77 ± 0.25 |
| 95 °C, 10’ | 32.15 ± 0.07 | 45.15 ± 0.28 |
| India | ||
| CB, 9 h | 65.53 ± 0.09 | 66.60 ± 0.36 |
| CB, 24 h | 65.61 ± 0.16 | 78.30 ± 0.34 |
| 86 °C, 4’ | 51.32 ± 0.08 | 53.38 ± 0.09 |
| 86 °C, 10’ | 61.58 ± 0.27 | 61.22 ± 0.17 |
| 95 °C, 4’ | 49.78 ± 0.13 | 62.12 ± 0.72 |
| 95 °C, 10’ | 52.93 ± 0.21 | 57.80 ± 0.56 |
| Peru | ||
| CB, 9 h | 38.97 ± 0.22 | 59.27 ± 0.20 |
| CB, 24 h | 41.88 ± 0.10 | 59.62 ± 0.15 |
| 86 °C, 4’ | 27.79 ± 0.15 | 44.73 ± 0.34 |
| 86 °C, 10’ | 27.23 ± 0.07 | 57.27 ± 0.23 |
| 95 °C, 4’ | 18.15 ± 0.30 | 53.98 ± 0.20 |
| 95 °C, 10’ | 28.68 ± 0.18 | 51.80 ± 0.35 |
| Rwanda | ||
| CB, 9 h | 39.26 ± 0.14 | 50.08 ± 0.20 |
| CB, 24 h | 49.01 ± 0.45 | 54.01 ± 0.07 |
| 86 °C, 4’ | 27.80 ± 0.13 | 49.38 ± 0.14 |
| 86 °C, 10’ | 29.75 ± 0.43 | 52.77 ± 0.20 |
| 95 °C, 4’ | 25.72 ± 0.22 | 49.25 ± 0.43 |
| 95 °C, 10’ | 47.32 ± 0.47 | 50.63 ± 0.30 |
| pH of Coffee Infusion | |||||
|---|---|---|---|---|---|
| infusion of unroasted beans | Brazil | Colombia | India | Peru | Rwanda |
| CB, 9 h | 6.04 | 5.75 | 6.00 | 5.17 | 5.16 |
| CB, 24 h | 5.92 | 6.11 | 6.24 | 6.17 | 6.44 |
| 86 °C, 4’ | 6.11 | 6.48 | 6.40 | 6.47 | 6.35 |
| 86 °C, 10’ | 6.06 | 6.28 | 6.20 | 5.94 | 6.50 |
| 95 °C, 4’ | 6.26 | 6.13 | 6.26 | 6.58 | 6.50 |
| 95 °C, 10’ | 6.16 | 6.32 | 6.29 | 6.23 | 6.35 |
| infusion of roasted beans | Brazil | Colombia | India | Peru | Rwanda |
| CB, 9 h | 5.25 | 5.07 | 5.62 | 5.12 | 4.99 |
| CB, 24 h | 5.31 | 5.07 | 5.71 | 5.19 | 5.21 |
| 86 °C, 4’ | 5.15 | 5.07 | 5.50 | 5.05 | 5.17 |
| 86 °C, 10’ | 5.14 | 5.03 | 5.48 | 5.07 | 5.14 |
| 95 °C, 4’ | 5.19 | 5.06 | 5.53 | 5.11 | 5.12 |
| 95 °C, 10’ | 5.20 | 5.07 | 5.50 | 5.02 | 5.10 |
| Country of the Beans Origins | Region | Roasting Degree |
|---|---|---|
| Brazil (Cerrado) | Cerrado Mineiro | medium |
| Colombia (Medellin) | Antioquia/Medellin | medium light |
| India (Monsooned Malabar) | Karnataka, Western Ghats | medium |
| Peru (Cepro Yanesha) | Villa Rica, Oxapampa, Pasco | medium dark |
| Rwanda (Sake) | Ngoma District, Eastern Province | light |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. https://doi.org/10.3390/molecules26123681
Muzykiewicz-Szymańska A, Nowak A, Wira D, Klimowicz A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules. 2021; 26(12):3681. https://doi.org/10.3390/molecules26123681
Chicago/Turabian StyleMuzykiewicz-Szymańska, Anna, Anna Nowak, Daria Wira, and Adam Klimowicz. 2021. "The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries" Molecules 26, no. 12: 3681. https://doi.org/10.3390/molecules26123681
APA StyleMuzykiewicz-Szymańska, A., Nowak, A., Wira, D., & Klimowicz, A. (2021). The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules, 26(12), 3681. https://doi.org/10.3390/molecules26123681