Eco-Friendly Nitrogen-Doped Graphene Preparation and Design for the Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological, Thermal Stability and Chemical Characterization
2.1.1. Morphological Analysis
2.1.2. Thermal and Elemental Analyses
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Investigation
2.1.4. X-ray Diffraction (XRD) Analysis
2.1.5. Raman Spectroscopy Investigations
2.2. ORR Performance Investigation
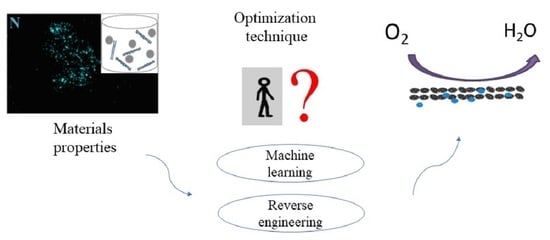
2.3. Machine Learning and Reverse Engineering for Materials Properties Optimization
3. Materials and Methods
3.1. Synthesis Section
3.2. Characterization
3.3. Modified Electrode Preparation
3.4. Machine Learning and Reverse Engineering Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Usachov, D.; Vilkov, O.; Grüneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-Doped Graphene: Efficient Growth, Structure, and Electronic Properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Tseng, C.; Lin, C.; Li, L.; Liu, H. Optical properties of nitrogen-doped graphene thin films probed by spectroscopic ellipsometry. Thin Solid Films 2014, 571, 675–679. [Google Scholar] [CrossRef]
- Miao, Q.; Wang, L.; Liu, Z.; Wei, B.; Xu, F.; Fei, W. Magnetic properties of N-doped graphene with high Curie temperature. Sci. Rep. 2016, 6, 21832. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- González, V.J.; Rodriguez, A.M.; León, V.; Frontiñán-Rubio, J.; Fierro, J.L.G.; Durán-Prado, M.; Munoz-Garcia, A.B.; Pavone, M.; Vázquez, E. Sweet graphene: Exfoliation of graphite and preparation of glucose-graphene cocrystals through mechanochemical treatments. Green Chem. 2018, 20, 3581–3592. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.-Y.; Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Dai, L.; Baek, J.-B. Large-Scale Production of Edge-Selectively Functionalized Graphene Nanoplatelets via Ball Milling and Their Use as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, H.; Qu, J.; Dai, L. Nitrogen-doped graphene by ball-milling graphite with melamine for energy conversion and storage. 2D Mater. 2015, 2, 44001. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Tan, J.; Wang, Q.; Wen, H.; Zhang, C. Nitrogen-doped graphene by all-solid-state ball-milling graphite with urea as a high-power lithium ion battery anode. J. Power Sources 2017, 342, 157–164. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Xi, H.; Yuan, R.; Zhang, C. First Direct Exfoliation and Nitrogen-Doping of Graphite to Graphene via Solid-State Pan Milling for High-Performance Lithium Ion Battery. Nano Adv. 2018, 3, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.; Müllen, K. Nitrogen-Doped Graphene and Its Iron-Based Composite as Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fugane, K.; Mori, T.; Niu, L.; Ye, J. Wet chemical synthesis of nitrogen-doped graphene towards oxygen reduction electrocatalysts without high-temperature pyrolysis. J. Mater. Chem. 2012, 22, 6575–6580. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.J.; Shen, P.K. Nitrogen-self-doped graphene-based non-precious metal catalyst with superior performance to Pt/C catalyst toward oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 3231. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A review of nitrogen-doped graphene catalysts for proton exchange membrane fuel cells-synthesis, characterization, and improvement. Nano-Struct. Nano-Objects 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Sun, X. Doped Graphene as Electrocatalysts for Oxygen Reduction Reaction. In Nanocarbons for Advanced Energy Conversion; Wiley: Hoboken, NJ, USA, 2015; pp. 17–42. [Google Scholar]
- Lu, X.; Wang, D.; Ge, L.; Xiao, L.; Zhang, H.; Liu, L.; Zhang, J.; An, M.; Yang, P. Enriched graphitic N in nitrogen-doped graphene as a superior metal-free electrocatalyst for the oxygen reduction reaction. New J. Chem. 2018, 42, 19665–19670. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Radu, T.; Biris, A.R.; Dan, M.; Voica, C.; Watanabe, F.; Vulcu, A. Platinum nanoparticles coated by graphene layers: A low-metal loading catalyst for methanol oxidation in alkaline media. J. Energy Chem. 2020, 40, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Vulcu, A.; Radu, T.; Porav, A.; Berghian-Grosan, C. Low-platinum catalyst based on sulfur doped graphene for methanol oxidation in alkaline media. Mater. Today Energy 2021, 19, 100588. [Google Scholar] [CrossRef]
- Chen, C.-M.; Zhang, Q.; Yang, M.-G.; Huang, C.-H.; Yang, Y.-G.; Wang, M.-Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Xiao, B.; Boudou, A.J.P.; Thomas, K.M. Reactions of Nitrogen and Oxygen Surface Groups in Nanoporous Carbons under Inert and Reducing Atmospheres. Langmuir 2005, 21, 3400–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials dur-ing pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.-Y.; Choi, H.-J.; Ju, M.J.; Choi, I.T.; Lim, K.; Ko, J.; Kim, H.K.; Kim, J.C.; Lee, J.-J.; Shin, D.; et al. Direct nitrogen fixation at the edges of graphene nanoplatelets as efficient electrocatalysts for energy conversion. Sci. Rep. 2013, 3, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, V. Nitrogen Doped Graphene as Potential Material for Hydrogen Storage. Graphene 2017, 6, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Childres, I.; Jaureguib, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman Spectroscopy of Graphene and Related Materials. In New Developments in Photon and Materials Research; Jang, J.I., Ed.; Nova Science Publishers: New York, NY, USA, 2013. [Google Scholar]
- Ni, Z.; Yu, T.; Luo, Z.Q.; Wang, Y.Y.; Liu, L.; Wong, C.P.; Miao, J.; Huang, W.; Shen, Z.X. Probing Charged Impurities in Suspended Graphene Using Raman Spectroscopy. ACS Nano 2009, 3, 569–574. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- 2018 CODATA Value: Faraday Constant. The NIST Reference on Constants, Units, and Uncertainty. Available online: https://physics.nist.gov/cgi-bin/cuu/Value?f (accessed on 23 June 2021).
- Tiwari, A.; Singh, V.; Mandal, D.; Nagaiah, T.C. Nitrogen containing carbon spheres as an efficient electrocatalyst for oxygen reduction: Microelectrochemical investigation and visualization. J. Mater. Chem. A 2017, 5, 20014–20023. [Google Scholar] [CrossRef]
- Pham, D.T.; Li, B.; Lee, Y.H. Nitrogen-doped activated graphene/SWCNT hybrid for oxygen reduction reaction. Curr. Appl. Phys. 2016, 16, 1242–1249. [Google Scholar] [CrossRef]
- Bai, X.; Shi, Y.; Guo, J.; Gao, L.; Wang, K.; Du, Y.; Ma, T. Catalytic activities enhanced by abundant structural defects and balanced N distribution of N-doped graphene in oxygen reduction reaction. J. Power Sources 2016, 306, 85–91. [Google Scholar] [CrossRef]
- Cui, H.J.; Yu, H.M.; Zheng, J.F.; Wang, Z.J.; Zhu, Y.Y.; Jia, S.P.; Jia, J.; Zhu, Z.P. N-Doped graphene frameworks with superhigh surface area: Excellent electrocatalytic performance for oxygen reduction. Nanoscale 2016, 8, 2795–2803. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Linc, J.; Ruoff, R.S. Exploration of the active cen-ter structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.; Ping, J.; Wang, Y.; Lin, T.; Huang, X.; Ma, Q.; Wang, F.; He, C.; Zhang, H. Electrochemical doping of three-dimensional graphene networks used as efficient electrocatalysts for oxygen reduction reaction. Nanoscale 2015, 7, 9394–9398. [Google Scholar] [CrossRef]
- Min, K.; Choi, B.; Park, K.; Cho, E. Machine learning assisted optimization of electrochemical properties for Ni-rich cathode materials. Sci. Rep. 2018, 8, 15778. [Google Scholar] [CrossRef] [Green Version]











| N-Doped Graphene | TGA/Weight Loss % | Elemental Analysis/Weight % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Regions | Residual C | N | O | C | H | ||||
| I | II | III | Total till 800 °C | ||||||
| N-Gr-3-24 | 3 | 3.2 | 7.3 | 19.4 | 80.6 | 9.61 | 9.31 | 80 | 1.08 |
| N-Gr-10-24 | 4 | 3.6 | 8.7 | 19.8 | 80.2 | 8.34 | 9.43 | 81.3 | 0.93 |
| N-Gr-3-48 | 4.6 | 2.5 | 13.9 | 28.4 | 71.6 | 13.1 | 12.98 | 72.5 | 1.42 |
| N-Gr-10-48 | 5.7 | 4.4 | 8.9 | 24.5 | 75.5 | 11.4 | 7.34 | 80.2 | 1.06 |
| Samples | Catalyst Loading (mg/cm2) | Onset Potential | Current at 1600 rpm/ Potential (mA/cm2) | Electron Transfer Number |
|---|---|---|---|---|
| N-Gr-3-24 (this work) | 0.14 | −0.16 V vs. Ag/AgCl | 4.7/−1 V vs. Ag/AgCl | 3.96 |
| N-aGS-800 [33] | 0.18 | 0.03 V vs. Ag/AgCl | 5.2/−1 V vs. Ag/AgCl | 3.99 |
| N-doped graphene (NG/Fe5.0) [11] | 0.05 | −0.04 V vs. Ag/AgCl | 3.8/−1 V vs. Ag/AgCl | 3.91 |
| N-doped graphene [34] | 0.152 | 0.82 V vs. RHE | 4.4/0 V vs. RHE | 3.9 |
| N-doped graphene [35] | 0.107 | 0.98 V vs. RHE | 3.9 at 1500 rpm/ −0.2 V vs. RHE | 3.9 |
| N-doped reduced graphene oxide (n-RGO-850 °C)[36] | 1.02 | −0.15 V vs. Ag/AgCl | 5.6/−1 V vs. Ag/AgCl | 3.35 |
| 3D-NB-doped graphene [37] | 0.082 | −0.06 V vs. Ag/agCl | 6/−0.9 V vs. Ag/AgCl | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, M.; Vulcu, A.; Porav, S.A.; Leostean, C.; Borodi, G.; Cadar, O.; Berghian-Grosan, C. Eco-Friendly Nitrogen-Doped Graphene Preparation and Design for the Oxygen Reduction Reaction. Molecules 2021, 26, 3858. https://doi.org/10.3390/molecules26133858
Dan M, Vulcu A, Porav SA, Leostean C, Borodi G, Cadar O, Berghian-Grosan C. Eco-Friendly Nitrogen-Doped Graphene Preparation and Design for the Oxygen Reduction Reaction. Molecules. 2021; 26(13):3858. https://doi.org/10.3390/molecules26133858
Chicago/Turabian StyleDan, Monica, Adriana Vulcu, Sebastian A. Porav, Cristian Leostean, Gheorghe Borodi, Oana Cadar, and Camelia Berghian-Grosan. 2021. "Eco-Friendly Nitrogen-Doped Graphene Preparation and Design for the Oxygen Reduction Reaction" Molecules 26, no. 13: 3858. https://doi.org/10.3390/molecules26133858
APA StyleDan, M., Vulcu, A., Porav, S. A., Leostean, C., Borodi, G., Cadar, O., & Berghian-Grosan, C. (2021). Eco-Friendly Nitrogen-Doped Graphene Preparation and Design for the Oxygen Reduction Reaction. Molecules, 26(13), 3858. https://doi.org/10.3390/molecules26133858