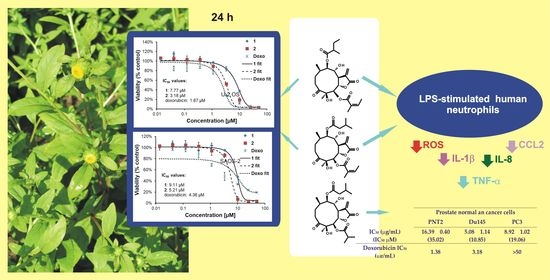
Germacranolides from Carpesium divaricatum: Some New Data on Cytotoxic and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
2.1. Identification of Isolated Sesquiterpene Lactones
2.2. Cytotoxic Activities of 1–3 against Selected Cancer Cell Lines
2.2.1. Viability of Cancer and Normal Cell Lines Treated with 3
2.2.2. Effects of 1 and 2 on Two Lines of Osteosarcoma with Different p53 Status
2.3. Effect of 1 and 2 on Lipopolysaccharide (LPS)-Stimulated Release of Proinflammatory Cytokines from Human Neutrophils
2.3.1. Cytotoxicity
2.3.2. Reactive Oxygen Species (ROS) Production
2.3.3. Release of Selected Proinflammatory Cytokines/Chemokines (IL-1β, IL-8, TNF-α, CCL2)
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Materials
4.3. Plant Material
4.4. Isolation and Identification of Gemacranolides from Aerial Parts of C. divaricatum
4.5. Cytotoxicity of 3 against Human Normal and Cancer Cell Lines
4.6. Cytotoxicity Assessment of 1 and 2 against U-2 OS and SAOS-2 Cell Lines
4.7. Anti-Inflammatory Activity of 1 and 2
4.7.1. Isolation of Human Neutrophils
4.7.2. Cytotoxicity Measurement
4.7.3. Reactive Oxygen Species (ROS) Production by Neutrophils
4.7.4. Proinflammatory Cytokine/Chemokine (IL-1β, IL-8, CCL-2 and TNFα) Production by LPS-Stimulated Neutrophils
4.7.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chang, L. Asteraceae. In Identification and Control of Common Weeds: Volume 3, 1st ed.; Zhejiang University Press: Hangzhou, China; Springer Nature: Singapore, 2017; pp. 651–659. [Google Scholar]
- Maruyama, M. Sesquiterpene lactones from Carpesium divaricatum. Phytochemistry 1990, 29, 547–550. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, K.R.; Zee, O.P. Sesquiterpene lactones from Carpesium divaricatum. Phytochemistry 1997, 46, 1245–1247. [Google Scholar] [CrossRef]
- Kim, D.K.; Baek, N.I.; Choi, S.U.; Lee, C.O.; Lee, K.R.; Zee, O.P. Four new cytotoxic germacranolides from Carpesium divaricatum. J. Nat. Prod. 1997, 60, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Si, J.-G.; Zhang, Q.-B.; Ding, G.; Zou, Z.-M. New highly oxygenated germacranolides from Carpesium divaricatum and their cytotoxic activity. Sci. Rep. 2016, 6, 27237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Si, J.-G.; Zhang, Q.-B.; Chen, J.-H.; Ding, G.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Three new highly oxygenated germacranolides from Carpesium divaricatum and their cytotoxic activity. Molecules 2018, 23, 1078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chen, J.-H.; Si, J.-G.; Ding, G.; Zhang, Q.-B.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Isolation, structure elucidation, and absolute configuration of germecrane isomers from Carpesium divaricatum. Sci. Rep. 2018, 8, 12418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Q.-B.; Fu, L.; Li, L.-Y.; Ma, L.-Y.; Si, J.-G.; Zhang, H.-W.; Wei, J.-H.; Yu, S.-S.; Zou, Z.-M. New antiproliferative germacranolides from Carpesium divaricatum. RSC Adv. 2019, 9, 11493–11502. [Google Scholar] [CrossRef]
- Zee, O.P.; Kim, D.K.; Choi, S.U.; Lee, C.O.; Lee, K.R. A new cytotoxic acyclic diterpene from Carpesium divaricatum. Arch. Pharm. Res. 1999, 22, 225–227. [Google Scholar]
- Zee, O.P.; Kim, D.K.; Lee, K.R. Thymol derivatives from Carpesium divaricatum. Arch. Pharm. Res. 1998, 21, 618–620. [Google Scholar] [CrossRef]
- Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. revisited: Newly identified constituents of the plant and their possible contribution to the biological activity of the plant. Molecules 2019, 24, 1614. [Google Scholar] [CrossRef] [Green Version]
- Wajs-Bonikowska, A.; Malarz, J.; Stojakowska, A. Composition of essential oils from roots and aerial parts of Carpesium divaricatum, a traditional herbal medicine and wild edible plant from South-East Asia, grown in Poland. Molecules 2019, 24, 4418. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mao, J.; Zhang, L.; Guo, H.; Yan, C.; Chen, M. Incaspitolide A isolated from Carpesium cernuum L. inhibits the growth of prostate cancer cells and induces apoptosis via regulation of the PI3K/Akt/xIAP pathway. Oncol. Lett. 2021, 21, 477. [Google Scholar] [CrossRef] [PubMed]
- Hehner, S.P.; Heinrich, M.; Bork, P.M.; Vogt, M.; Ratter, F.; Lehmann, V.; Schulze-Osthoff, K.; Dröge, W.; Schmitz, M.L. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκB-β. J. Biol. Chem. 1998, 273, 1288–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef] [Green Version]
- Koch, E.; Klaas, C.A.; Rüngeler, P.; Castro, V.; Morad, G.; Vichnewski, W.; Merfort, I. Inhibition of inflammatory cytokine production and lymphocyte proliferation by structurally different sesquiterpene lactones correlates with their effect on activation of NF-κB. Biochem. Pharmacol. 2001, 62, 795–801. [Google Scholar] [CrossRef]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-κB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.J.; Heilmann, J. Quantitative structure-cytotoxicity relationships of sesquiterpene lactones derived from partial charge (Q)-based fractional accessible surface area descriptors (Q_frASAs). Mol. Inf. 2002, 21, 276–287. [Google Scholar] [CrossRef]
- Scotti, M.T.; Fernandes, M.B.; Ferreira, M.J.P.; Emerenciano, V.P. Quantitative structure–activity relationship of sesquiterpene lactones with cytotoxic activity. Bioorg. Med. Chem. 2007, 15, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hofmann, A.; Siedle, B.; Terfloth, L.; Merfort, I.; Gasteiger, J. Development of a structural model for NF-KB inhibition of sesquiterpene lactones using self-organizing neural networks. J. Med. Chem. 2006, 49, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Structure-activity and activity-activity relationships of sesquiterpene lactones. In Sesquiterpene lactones; Sülsen, V.P., Martino, V.S., Eds.; Springer: Cham, Switzerland, 2018; pp. 349–371. [Google Scholar] [CrossRef]
- Kim, E.J.; Jin, H.K.; Kim, Y.K.; Lee, H.Y.; Lee, S.Y.; Lee, K.R.; Zee, O.P.; Han, J.W.; Lee, H.W. Suppression by a sesquiterpene lactone from Carpesium divaricatum of inducible nitric oxide synthase by inhibiting nuclear factor-κB activation. Biochem. Pharmacol. 2001, 61, 903–910. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, H.; Kwon, J.; Li, H.; Lee, D.Y.; Lim, H.J.; Kim, M.-R.; Moon, D.-C.; Ryu, J.-H. A germacranolide sesquiterpene lactone suppressed inducible nitric oxide synthase by downregulating NF-κB activity. Can. J. Physiol. Pharmacol. 2011, 89, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-I. Compounds with antiproliferative activity on five human cancer cell lines from South Korean Carpesium triste. Nat. Prod. Commun. 2012, 7, 825–826. [Google Scholar] [CrossRef] [Green Version]
- Goswami, A.C.; Baruah, R.N.; Sharma, R.P.; Baruah, J.N.; Kulanthaivel, P.; Herz, W. Germacranolides from Inula cappa. Phytochemistry 1984, 23, 367–372. [Google Scholar] [CrossRef]
- Gao, X.; Lin, C.-J.; Jia, Z.-J. Cytotoxic germacranolides and acyclic diterpenoids from the seeds of Carpesium triste. J. Nat. Prod. 2007, 70, 830–834. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.P.; Bald, A.; Cassatella, M. Activation of NF-κB pathway by inflammatory stimuli in human neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef]
- Gopal, Y.N.V.; Chanchorn, E.; Van Dyke, M.W. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol. Cancer Ther. 2009, 8, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H.; Al Kury, L.T. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing p53-dependent apoptosis and inhibiting VEGF expression. Biomed. Pharmacother. 2018, 107, 1488–1495. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, P.; Zhang, H.; Liu, B.; Shi, Y. P53 is required for Doxorubicin-induced apoptosis via the TGF-beta signalling pathway in osteosarcoma-derived cells. Am. J. Cancer Res. 2016, 6, 114–125. [Google Scholar] [PubMed]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Hwang, B.Y.; Jeong, E.-S.; Lee, Y.-M.; Yoo, H.-S.; Chung, Y.-B.; Hong, J.T.; Moon, D.-C. Cytotoxic germacranolide sesquiterpene lactones from Carpesium triste var. manshuricum. Arch. Pharm. Res. 2007, 30, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, K.; Podolak, I.; Galanty, A.; Żmudzki, P.; Koczurkiewicz, P.; Piska, K.; Pękala, E.; Janeczko, Z. Two new triterpenoid saponins from the leaves of Impatiens parviflora DC. and their cytotoxic activity. Ind. Crop. Prod. 2017, 96, 71–79. [Google Scholar] [CrossRef]






| Concentration (µg/mL) | Cell Viability (%) ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| Prostate Normal and Cancer Cells | Keratinocytes and Melanoma Cells | Colon Cancer | ||||||
| PNT2 | Du145 | PC3 | HaCaT | A375 | HTB140 | HT29 | Caco-2 | |
| 1 | 87.09 ± 1.79 | 68.42 ± 0.52 | 78.51 ± 1.08 | 86.79 ± 1.70 | 64.22 ± 1.13 | 81.37 ± 1.28 | 69.27 ± 1.75 | 52.79 ± 1.62 |
| 3 | 77.17 ± 1.32 | 56.28 ± 0.97 | 65.03 ± 1.46 | 74.50 ± 2.29 | 55.42 ± 1.44 | 70.79 ± 2.37 | 57.95 ± 1.66 | 38.59 ± 2.35 |
| 5 | 69.39 ± 0.97 | 44.83 ± 2.05 | 49.91 ± 2.02 | 64.39 ± 1.33 | 43.77 ± 1.69 | 50.92 ± 1.80 | 49.25 ± 2.00 | 15.05 ± 2.91 |
| 10 | 52.84 ± 2.31 | 37.72 ± 2.16 | 37.21 ± 1.27 | 30.51 ± 1.32 | 32.99 ± 2.30 | 21.49 ± 2.53 | 38.00 ± 2.29 | 1.96 ± 2.70 |
| 20 | 32.45 ± 1.84 | 23.62 ± 1.54 | 29.19 ± 1.89 | 0.79 ± 0.72 | 7.09 ± 1.78 | 4.10 ± 1.75 | 24.42 ± 1.10 | 0.31 ± 0.43 |
| 30 | 21.40 ± 0.45 | 12.49 ± 1.94 | 5.42 ± 2.25 | 0 ± 0 | 0.82 ± 0.65 | 0.59 ± 0.78 | 10.38 ± 1.09 | 0 ± 0 |
| 50 | 3.29 ± 1.27 | 0.62 ± 0.58 | 0.65 ± 0.33 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.79 ± 0.78 | 0 ± 0 |
| IC50 (IC50 µM) | 16.39 ± 0.40(35.02) | 5.08 ± 1.14 (10.85) | 8.92 ± 1.02 (19.06) | 7.95 ± 0.15 (16.99) | 9.57 ± 0.87 (20.45) | 6.36 ± 0.38 (13.59) | 6.23 ± 0.89 (13.31) | 3.88 ± 0.10 (8.29) |
| Doxorubicin IC50 | 1.38 ± 0.10 | 3.18 ± 0.10 | >50 | >4.68 ± 0.07 | 0.59 ± 0.04 | >5.71 ± 0.05 | 1.53 ± 0.15 | 3.44 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Skalniak, Ł.; Galanty, A.; Kiss, A.K.; Stojakowska, A. Germacranolides from Carpesium divaricatum: Some New Data on Cytotoxic and Anti-Inflammatory Activity. Molecules 2021, 26, 4644. https://doi.org/10.3390/molecules26154644
Kłeczek N, Malarz J, Gierlikowska B, Skalniak Ł, Galanty A, Kiss AK, Stojakowska A. Germacranolides from Carpesium divaricatum: Some New Data on Cytotoxic and Anti-Inflammatory Activity. Molecules. 2021; 26(15):4644. https://doi.org/10.3390/molecules26154644
Chicago/Turabian StyleKłeczek, Natalia, Janusz Malarz, Barbara Gierlikowska, Łukasz Skalniak, Agnieszka Galanty, Anna K. Kiss, and Anna Stojakowska. 2021. "Germacranolides from Carpesium divaricatum: Some New Data on Cytotoxic and Anti-Inflammatory Activity" Molecules 26, no. 15: 4644. https://doi.org/10.3390/molecules26154644
APA StyleKłeczek, N., Malarz, J., Gierlikowska, B., Skalniak, Ł., Galanty, A., Kiss, A. K., & Stojakowska, A. (2021). Germacranolides from Carpesium divaricatum: Some New Data on Cytotoxic and Anti-Inflammatory Activity. Molecules, 26(15), 4644. https://doi.org/10.3390/molecules26154644