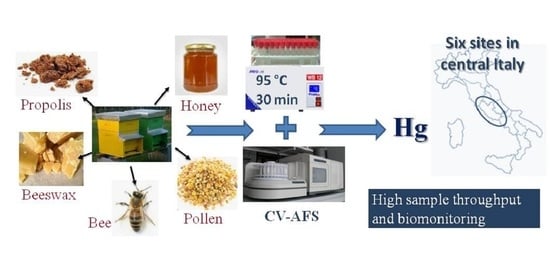
An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison with Previous Methods
2.2. Linearity and Selectivity
2.3. Detection and Quantification Limits
2.4. Accuracy and Precision
2.5. Hg Concentrations in Real Samples
3. Materials and Methods
3.1. Study Areas and Sample Collection
3.2. Materials and Reagents
3.3. Sample Preparation and Analysis
3.4. Quality Assurance
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Langford, N.J.; Ferner, R.E. Toxicity of mercury. J. Hum. Hypertens. 1999, 13, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Selin, N.E. Global biogeochemical cycling of mercury: A review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef] [Green Version]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013; Available online: https://wedocs.unep.org/handle/20.500.11822/7984 (accessed on 2 May 2021).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury; Department of Health and Human Services: Atlanta, GA, USA, 1999. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf (accessed on 2 May 2021).
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, O.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Health 2005, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mercury and Health Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 2 May 2021).
- European Union. European Food Safety Authority. Mercury as undesirable substance in animal feed. Scientific opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 654, 1–74. [Google Scholar]
- European Union. European Food Safety Authority (EFSA). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- European Union. European Parliament. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off. J. Eur. Communities 2004, 23, 3–16. [Google Scholar]
- European Union. European Parliament. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off. J. Eur. Union 2008, 152, 1–44. [Google Scholar]
- European Union. European Parliament. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control). Off. J. Eur. Union 2010, 334, 17–119. [Google Scholar]
- European Union. European Parliament. Regulation (EU) 2017/852 of the European Parliament and of the Council of 17 May 2017 on mercury, and repealing Regulation (EC) No 1102/2008. Off. J. Eur. Union 2017, 137, 1–21. [Google Scholar]
- Pirrone, N.; Aas, W.; Cinnirella, S.; Ebinghaus, R.; Hedgecock, I.M.; Pacyna, J.; Sprovieri, F.; Sunderland, E.M. Toward the next generation of air quality monitoring: Mercury. Atmos. Environ. 2013, 80, 599–611. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Ebinghaus, R.; Kock, H.; Dommergue, A. A review of worldwide atmospheric mercury measurements. Atmos. Chem. Phys. Discuss. 2010, 10, 8245–8265. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Wolterbeek, H.T. Biomonitoring of trace element air pollution: Principles, possibilities and perspectives. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef]
- Bargagli, R. Moss and lichen biomonitoring of atmospheric mercury: A review. Sci. Total Environ. 2016, 572, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, L.; Candotto Carniel, F.; Capozzi, F.; Tretiach, M. Congruence evaluation of mercury pollution patterns around a waste incinerator over a 16-year-long period using different biomonitors. Atmosphere 2019, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Massimi, L.; Conti, M.E.; Mele, G.; Ristorini, M.; Astolfi, M.L.; Canepari, S. Lichen transplants as indicators of atmospheric element concentrations: A high spatial resolution comparison with PM 10 samples in a polluted area (Central Italy). Ecol. Indic. 2019, 101, 759–769. [Google Scholar] [CrossRef]
- Vitali, M.; Antonucci, A.; Owczarek, M.; Guidotti, M.; Astolfi, M.L.; Manigrasso, M.; Avino, P.; Bhattacharya, B.; Protano, C. Air quality assessment in different environmental scenarios by the determination of typical heavy metals and Persistent Organic Pollutants in native lichen Xanthoria parietina. Environ. Pollut. 2019, 254, 113013. [Google Scholar] [CrossRef] [PubMed]
- Ristorini, M.; Astolfi, M.L.; Frezzini, M.A.; Canepari, S.; Massimi, L. Evaluation of the efficiency of Arundo donax L. Leaves as biomonitors for atmospheric element concentrations in an urban and industrial area of central Italy. Atmosphere 2020, 11, 226. [Google Scholar] [CrossRef] [Green Version]
- Bargańska, Z.; Ślebioda, M.; Namieśnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Maragou, N.C.; Pavlidis, G.; Karasali, H.; Hatjina, F. Cold vapor atomic absorption and microwave digestion for the determination of mercury in honey, pollen, propolis and bees of greek origin. Glob. NEST J. 2016, 18, 690–696. [Google Scholar]
- Grainger, M.N.C.; Hewitt, N.; French, A.D. Optimised approach for small mass sample preparation and elemental analysis of bees and bee products by inductively coupled plasma mass spectrometry. Talanta 2020, 214, 120858. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis. Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemical constituents of selected unifloral Greek bee-honeys with antimicrobial activity. Food Chem. 2011, 129, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef]
- Toporcák, J.; Legáth, J.; Kul’ková, J. Levels of mercury in samples of bees and honey from areas with and without industrial contamination. Vet. Med. 1992, 37, 405–412. [Google Scholar]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Dos Santos Depoi, F.; Bentlin, F.R.S.; Pozebon, D. Methodology for Hg determination in honey using cloud point extraction and cold vapour-inductively coupled plasma optical emission spectrometry. Anal. Methods 2010, 2, 180–185. [Google Scholar] [CrossRef]
- Domínguez, M.A.; Grünhut, M.; Pistonesi, M.F.; Di Nezio, M.S.; Centuriόn, M.E. Automatic flow-batch system for cold vapor atomic absorption spectroscopy determination of mercury in honey from argentina using online sample treatment. J. Agric. Food Chem. 2012, 60, 4812–4817. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.-M.; Feng, Q.; He, J.-Z. Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chem. Toxicol. 2013, 53, 256–262. [Google Scholar] [CrossRef]
- Bilandžić, N.; Gačić, M.; Ðokić, M.; Sedak, M.; Šipušić, Ð.I.; Končurat, A.; Gajger, I.T. Major and trace elements levels in multifloral and unifloral honeys in Croatia. J. Food Compos. Anal. 2014, 33, 132–138. [Google Scholar] [CrossRef]
- Vieira, H.P.; Nascentes, C.C.; Windmöller, C.C. Development and comparison of two analytical methods to quantify the mercury content in honey. J. Food Compos. Anal. 2014, 34, 1–6. [Google Scholar] [CrossRef]
- Meli, M.A.; Desideri, D.; Roselli, C.; Benedetti, C.; Feduzi, L. Essential and toxic elements in honeys from a region of central Italy. J. Toxicol. Environ. Health 2015, 78, 617–627. [Google Scholar] [CrossRef]
- Toth, T.; Kopernicka, M.; Sabo, R.; Kopernicka, T. The evaluation of mercury in honey bees and their products from eastern Slovakia. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 257–260. [Google Scholar]
- Dżugan, M.; Wesołowska, M.; Zaguła, G.; Kaczmarski, M.; Czernicka, M.; Puchalski, C. Honeybees (Apis mellifera) as a biological barrier for contamination of honey by environmental toxic metals. Environ. Monit. Assess. 2018, 190, 101. [Google Scholar] [CrossRef]
- Jovetić, M.S.; Redžepović, A.S.; Nedić, N.M.; Vojt, D.; Đurđić, S.Z.; Brčeski, I.D.; Milojković-Opsenica, D.M. Urban honey—The aspects of its safety. Arh. Hig. Rada Toksikol. 2018, 69, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Bommuraj, V.; Chen, Y.; Klein, H.; Sperling, R.; Barel, S.; Shimshoni, J.A. Pesticide and trace element residues in honey and beeswax combs from Israel in association with human risk assessment and honey adulteration. Food Chem. 2019, 299, 125123. [Google Scholar] [CrossRef] [PubMed]
- Roman, A. Concentration of chosen trace elements of toxic properties in bee pollen loads. Pol. J. Environ. Stud. 2009, 18, 265–272. [Google Scholar]
- Morgano, M.A.; Martins, M.C.T.; Rabonato, L.C.; Milani, R.F.; Yotsuyanagi, K.; Rodriguez-Amaya, D.B. Inorganic contaminants in bee pollen from southeastern Brazil. J. Agric. Food Chem. 2010, 58, 6876–6883. [Google Scholar] [CrossRef] [PubMed]
- Cvek, J.; Medić-Šarić, M.; Vitali, D.; Vedrina-Dragojević, I.; Šmit, Z.; Tomić, S. The content of essential and toxic elements in Croatian propolis samples and their tinctures. J. Apicult. Res. 2008, 47, 35–45. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Bermejo, F.J.O. Element content of propolis collected from different areas of South Spain. Environ. Monit. Assess. 2013, 185, 6035–6047. [Google Scholar] [CrossRef]
- Matin, G.; Kargar, N.; Buyukisik, H.B. Bio-monitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecol. Eng. 2016, 90, 331–335. [Google Scholar] [CrossRef]
- Perugini, M.; Manera, M.; Grotta, L.; Abete, M.C.; Tarasco, R.; Amorena, M. Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: Honeybees as bioindicators. Biol. Trace Elem. Res. 2011, 140, 170–176. [Google Scholar] [CrossRef]
- Zaric, N.M.; Deljanin, I.; Ilijević, K.; Stanisavljević, L.; Ristić, M.; Gržetić, I. Assessment of spatial and temporal variations in trace element concentrations using honeybees (Apis mellifera) as bioindicators. PeerJ 2018, 6, e5197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astolfi, M.L.; Conti, M.E.; Marconi, E.; Massimi, L.; Canepari, S. Effectiveness of different sample treatments for the elemental characterization of bees and beehive products. Molecules 2020, 25, 4263. [Google Scholar] [CrossRef]
- Melaku, S.; Gelaude, I.; Vanhaecke, F.; Moens, L.; Dams, R. Comparison of pyrolysis and microwave acid digestion techniques for the determination of mercury in biological and environmental materials. Microchim. Acta 2003, 142, 7–12. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marconi, E.; Protano, C.; Canepari, S. Comparative elemental analysis of dairy milk and plant-based milk alternatives. Food Control 2020, 116, 107327. [Google Scholar] [CrossRef]
- Schlathauer, M.; Reitsam, V.; Schierl, R.; Leopold, K. A new method for quasi-reagent-free biomonitoring of mercury in human urine. Anal. Chim. Acta 2017, 965, 63–71. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Protano, C.; Schiavi, E.; Marconi, E.; Capobianco, D.; Massimi, L.; Ristorini, M.; Baldassarre, M.E.; Laforgia, N.; Vitali, M.; et al. A prophylactic multi-strain probiotic treatment to reduce the absorption of toxic elements: In-vitro study and biomonitoring of breast milk and infant stools. Environ. Int. 2019, 130, 104818. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Pietris, G.; Mazzei, C.; Marconi, E.; Canepari, S. Element levels and predictors of exposure in the hair of Ethiopian children. Int. J. Environ. Res. Public Health 2020, 17, 8652. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.; Alves, C.N.; Morte, E.S.B.; Júnior, A.D.F.S.; Araujo, R.G.O.; Santos, D.C.M.B. Determination of essential and potentially toxic elements and their estimation of bioaccessibility in honeys. Microchem. J. 2019, 151, 104221. [Google Scholar] [CrossRef]
- Tenaglia, H.; Venturini, E.; Raffaelli, R. Linee guida per la validazione dei metodi analitici e per il calcolo dell’incertezza di misura. In Manuali ARPA; Agenzia Regionale Prevenzione e Ambiente dell’Emilia Romagna: Bologna, Italy, 2003. [Google Scholar]
- European Commission. Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (notified under document number C(2002) 3044) (Text with EEA relevance). Off. J. Eur. Communities 2002, 221, 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002D0657&rid=13 (accessed on 3 August 2021).
- Astolfi, M.L.; Protano, C.; Marconi, E.; Piamonti, D.; Massimi, L.; Brunori, M.; Vitali, M.; Canepari, S. Simple and rapid method for the determination of mercury in human hair by cold vapour generation atomic fluorescence spectrometry. Microchem. J. 2019, 150, 104186. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. The use of honeybees and honey as environmental bioindicators for metals and radionuclides: A review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
- AL-Alam, J.; Chbani, A.; Faljoun, Z.; Millet, M. The use of vegetation, bees, and snails as important tools for the biomonitoring of atmospheric pollution—A review. Environ. Sci. Pollut. Res. 2019, 26, 9391–9408. [Google Scholar] [CrossRef]
- Zhelyazkova, I. Honeybees—Bioindicators for environmental quality. Bulg. J. Agric. Sci. 2012, 18, 435–442. [Google Scholar]
- Finger, D.; Filho, I.K.; Torres, Y.R.; Quináia, S.P. Propolis as an indicator of environmental contamination by metals. Bull. Environ. Contam. Toxicol. 2014, 92, 259–264. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and their products as potential bioindicators of heavy metals contamination. Environ. Monit. Assess. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Codex Alimentarius. Standard for Honey (CXS 12-1981). Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 2 May 2021).
- Joint FAO; World Health Organization; WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]

| Calibration Standards | Parameter a | ||||
|---|---|---|---|---|---|
| a | s(a) | b | s(b) | R2 | |
| Aqueous standards | 9.68 × 10 | 9.68 × 10 | 1.86 × 104 | 1.16 × 103 | 0.999 |
| Bee-addition standards | 1.32 × 102 | 3.14 × 10 | 1.64 × 104 | 2.30 × 103 | 0.999 |
| Beeswax-addition standards | 7.65 × 10 | 1.05 × 10 | 1.71 × 104 | 2.22 × 103 | 0.999 |
| Honey-addition standards | 1.07 × 102 | 1.34 × 102 | 1.61 × 104 | 2.05 × 103 | 0.999 |
| Honeydew-addition standards | 1.38 × 102 | 8.58 ×10 | 1.65 × 104 | 1.78 × 103 | 0.999 |
| Pollen-addition standards | 1.30 × 102 | 3.73 × 10 | 1.62 × 104 | 2.07 × 103 | 0.998 |
| Propolis-addition standards | 3.85 × 102 | 2.50 × 102 | 1.76 × 104 | 1.40 × 103 | 0.999 |
| Royal jelly-addition standards | 7.65 × 10 | 1.05 × 10 | 1.80 × 104 | 9.19 × 102 | 0.998 |
| Low Level Spike (0.02 µg L−1) | Intermediate Level Spike (0.2 µg L−1) | High Level Spike (1 µg L−1) | |||||
|---|---|---|---|---|---|---|---|
| Matrix | Mass (g) | R% | CV% | R% | CV% | R% | CV% |
| Honey | 0.05 | 86 | 5.4 | 116 | 9.3 | 96 | 7.8 |
| Honeydew | 0.1 | 89 | 0.4 | 113 | 10 | 91 | 8.4 |
| Pollen | 0.02 | 92 | 9.6 | 90 | 3.7 | 95 | 3.6 |
| Propolis | 0.02 | 104 | 9.8 | 98 | 8.6 | 91 | 2.5 |
| Beeswax | 0.05 | 92 | 10 | 111 | 8.5 | 99 | 2.0 |
| Royal Jelly | 0.02 | 117 | 9.3 | 108 | 4.4 | 110 | 0.9 |
| Bees | 0.02 | 95 | 8.9 | 97 | 4.5 | 91 | 10 |
| Matrix | Mass a (g) | Digestion Time a (min) |
|---|---|---|
| Bees | 0.02–0.2 | 30 or 60 |
| Beeswax | 0.02 | 60 |
| 0.05–0.1 | 30 or 60 | |
| 0.2 | 60 | |
| Honey | 0.05 | 30 or 60 |
| 0.1 | 60 | |
| Honeydew | 0.05 | 60 |
| 0.1 | 30 or 60 | |
| 0.2 | 60 | |
| Pollen | 0.02–0.2 | 30 or 60 |
| Propolis | 0.02 | 30 or 60 |
| Royal Jelly | 0.02–0.2 | 30 or 60 |
| Matrix | Statistics | OR | FAI | MC | MG | MS | TR |
|---|---|---|---|---|---|---|---|
| Honey | N | 14 | 6 | 10 | 10 | 10 | 4 |
| Mean | 0.91 a,b,c,d,e | 2.26 a | 2.68 b | 2.66 c | 2.43 d | 3.37 e | |
| SD | 0.23 | 0.69 | 0.75 | 0.36 | 0.60 | 0.60 | |
| Median | 0.78 | 1.92 | 2.97 | 2.53 | 2.07 | 3.37 | |
| Minimum | 0.66 | 1.80 | 1.35 | 2.30 | 1.95 | 2.95 | |
| Maximum | 1.25 | 3.06 | 3.17 | 3.23 | 3.20 | 3.80 | |
| Pollen | N | 14 | NS | 12 | 10 | 4 | 4 |
| Mean | 3.2 a,b | - | 7.6 | 7.5 | 12.8 a | 10.4 b | |
| SD | 1.4 | - | 2.0 | 1.9 | 8.0 | 2.5 | |
| Median | 3.0 | - | 7.2 | 7.0 | 12.8 | 10.4 | |
| Minimum | <3 | - | 5.1 | 5.9 | 7.2 | 8.7 | |
| Maximum | 5.6 | - | 10.2 | 10.6 | 18.5 | 12.2 | |
| Propolis | N | 10 | NS | NS | 4 | 6 | NS |
| Mean | 4.6 a | - | - | 7.54 b | 14.8 a,b | - | |
| SD | 1.2 | - | - | 0.65 | 2.1 | - | |
| Median | 4.8 | - | - | 7.54 | 15.7 | - | |
| Minimum | <3 | - | - | 7.08 | 12.4 | - | |
| Maximum | 5.7 | - | - | 8.00 | 16.4 | - | |
| Beeswax | N | 14 | 8 | 14 | 12 | 12 | 4 |
| Mean | 2.8 a | 5.9 | 6.4 | 4.8 | 4.9 | 11.5 a | |
| SD | 1.6 | 2.8 | 3.0 | 1.7 | 3.1 | 1.8 | |
| Median | 2.8 | 5.2 | 4.5 | 4.2 | 3.9 | 11.5 | |
| Minimum | <1 | 3.5 | 3.3 | 3.5 | 3.0 | 10.2 | |
| Maximum | 5.7 | 9.5 | 10.6 | 8.1 | 11.2 | 12.7 | |
| Bees | N | 14 | 6 | 14 | 14 | 10 | 4 |
| Mean | 1.76 a,b,c | 16.2 a | 11.0 | 16.2 b | 17.1 c | 14.5 | |
| SD | 0.85 | 2.7 | 5.8 | 5.6 | 8.5 | 5.2 | |
| Median | 2.09 | 15.1 | 10.4 | 14.5 | 17.0 | 14.5 | |
| Minimum | 0.53 | 14.4 | 1.6 | 8.3 | 9.1 | 10.9 | |
| Maximum | 2.65 | 19.3 | 20.3 | 25.3 | 31.0 | 18.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, M.L.; Conti, M.E.; Ristorini, M.; Frezzini, M.A.; Papi, M.; Massimi, L.; Canepari, S. An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry. Molecules 2021, 26, 4878. https://doi.org/10.3390/molecules26164878
Astolfi ML, Conti ME, Ristorini M, Frezzini MA, Papi M, Massimi L, Canepari S. An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry. Molecules. 2021; 26(16):4878. https://doi.org/10.3390/molecules26164878
Chicago/Turabian StyleAstolfi, Maria Luisa, Marcelo Enrique Conti, Martina Ristorini, Maria Agostina Frezzini, Marco Papi, Lorenzo Massimi, and Silvia Canepari. 2021. "An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry" Molecules 26, no. 16: 4878. https://doi.org/10.3390/molecules26164878
APA StyleAstolfi, M. L., Conti, M. E., Ristorini, M., Frezzini, M. A., Papi, M., Massimi, L., & Canepari, S. (2021). An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry. Molecules, 26(16), 4878. https://doi.org/10.3390/molecules26164878