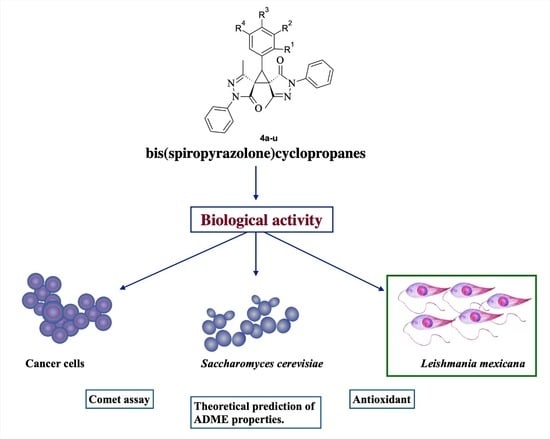
Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Biological Activity of Bis(spiro pyrazolone)cyclopropanes against Yeast S. cerevisiae, and Human Cancer Cell Lines
2.3. Evaluation of Cytotoxic and Leishmanicidal Activity
2.4. Evaluation of Other Activities: Antioxidant and Genotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General
4.1.2. General Procedure for the Synthesis of 4,4′-(Arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) 2a-u
4.1.3. Synthesis of (5R*,6R*)-11-Aryl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.4.1]undeca-3,9-diene-1,7-diones 4
4.2. Biological Evaluation
4.2.1. Evaluation of the Fungicidal Activity on S. cerevisiae
4.2.2. Drop Test
4.2.3. Cell Culture Procedures
4.2.4. Cell Viability Analysis and Determination of the Inhibitory Concentration 50 (IC50) on Mammalian Cancer Cells Lines
4.2.5. Cell Viability Analysis by Fluorescein Diacetate/Ethidium Bromide
4.2.6. Comet Assay
4.2.7. Statistical Analyses
4.2.8. DPPH Radical Scavenging Assay
4.2.9. Evaluation of Leishmanicidal Activity
4.2.10. Evaluation of the Cell Viability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Jamwal, A.; Javed, A.; Bhardwaj, V. A review on Pyrazole derivatives of pharmacological potential. J. Pharm. Biosci. 2013, 3, 114–123. [Google Scholar]
- Dewangan, D.; Kumar, T.; Alexander, A.; Nagori, K.; Tripathi, D.K. Pyrazole: Their Chemistry and Pharmacological Potentials: A Review. Curr. Pharma Res. 2011, 1, 369–377. [Google Scholar]
- Pavlov, P.T.; Goleneva, A.F.; Lesnov, A.E.; Prokhorova, T.S. Biological Activity of Some Pyrazolone Derivatives. Pharm. Chem. J. 1998, 32, 370–372. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, O.; Nawaz, F.; Alam, J.; Alam, P. Current status of pyrazole and its biological activities. J. Pharm. Bioallied Sci. 2016, 8, 2–17. [Google Scholar] [CrossRef]
- Higashi, Y.; Jitsuiki, D.; Chayama, K.; Yoshizumi, M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Niknam, K.; Mirzaee, S. Silica Sulfuric Acid, an Efficient and Recyclable Solid Acid Catalyst for the Synthesis of 4,4′-(Arylmethylene)bis(1H-pyrazol-5-ols). Synth. Commun. 2011, 41, 2403–2413. [Google Scholar] [CrossRef]
- Torrence, P.F.; Fan, X.; Zhang, X.; Loiseau, P.M. Structurally diverse 5-substituted pyrimidine nucleosides as inhibitors of Leishmania donovani promastigotes in vitro. Bioorg. Med. Chem. Lett. 2006, 16, 5047–5051. [Google Scholar] [CrossRef]
- Gehrke, S.S. Small Molecules with Anti-Trypanosomal and Anti-Leishmanial Activity; University of East Anglia: Norwich, UK, 2012. [Google Scholar]
- Sharma, G.; Chowdhury, S.; Sinha, S.; Majumder, H.K.; Kumar, S.V. Antileishmanial activity evaluation of bis-lawsone analogs and DNA topoisomerase-I inhibition studies. J. Enzyme Inhib. Med. Chem. 2014, 29, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharate, S.B.; Bharate, J.B.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Mudududdla, R.; Yadav, R.R.; Singh, B.B.; Sharma, P.R.; Maity, S.; et al. Discovery of 3,3′-diindolylmethanes as potent antileishmanial agents. Eur. J. Med. Chem. 2013, 63, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadena-Cruz, J.E.; Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Bailon-Moscoso, N.; Murillo-Sotomayor, K.E.; Ortiz-Guamán, N.V.; Heredia-Moya, J. Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chem. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Ajay Kumar, K. Brief review on cyclopropane analogs: Synthesis and their pharmacological applications. Int. J. Pharm. Pharm. Sci. 2013, 5, 454–459. [Google Scholar]
- Salaün, J.; Bird, M.S. Biologically active cyclopropanes and cyclopropenes. Curr. Med. Chem. 1995, 2, 511–542. [Google Scholar]
- Matsui, K.; Matsui, K.; Kan, Y.; Kikuchi, J.; Matsushima, K.; Takemura, M.; Maki, H.; Kozono, I.; Ueda, T.; Minagawa, K. Stalobacin: Discovery of Novel Lipopeptide Antibiotics with Potent Antibacterial Activity against Multidrug-Resistant Bacteria. J. Med. Chem. 2020, 63, 6090–6095. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-F.; Huang, P.; Zhang, D.; Wang, Q.; Zheng, N.; Wu, R.; Liu, Q.; Jin, L.-X.; Yu, X.-H.; Ji, X.-H.; et al. 1-(3-Amino-4-morpholino-1H-indazole-1-carbonyl)-N-phenylcyclopropane-1-carboxamide: Design, synthesis, crystal structure, antitumor activity, DFT and Hirshfeld surface analysis. J. Mol. Struct. 2020, 1210, 127996. [Google Scholar] [CrossRef]
- Arita, T.; Asano, M.; Kubota, K.; Domon, Y.; Machinaga, N.; Shimada, K. Discovery of DS34942424: An orally potent analgesic without mu opioid receptor agonist activity. Bioorg. Med. Chem. 2020, 28, 115714. [Google Scholar] [CrossRef]
- Fioravanti, R.; Romanelli, A.; Mautone, N.; Di Bello, E.; Rovere, A.; Corinti, D.; Zwergel, C.; Valente, S.; Rotili, D.; Botrugno, O.A.; et al. Tranylcypromine-Based LSD1 Inhibitors: Structure-Activity Relationships, Antiproliferative Effects in Leukemia, and Gene Target Modulation. ChemMedChem 2020, 15, 643–658. [Google Scholar] [CrossRef]
- Qian, P.; Du, B.; Song, R.; Wu, X.; Mei, H.; Han, J.; Pan, Y. N-Iodosuccinimide-Initiated Spirocyclopropanation of Styrenes with 1,3-Dicarbonyl Compound for the Synthesis of Spirocyclopropanes. J. Org. Chem. 2016, 81, 6546–6553. [Google Scholar] [CrossRef]
- Schobert, R.; Knauer, S.; Seibt, S.; Biersack, B. Anticancer active illudins: Recent developments of a potent alkylating compound class. Curr. Med. Chem. 2011, 18, 790–807. [Google Scholar] [CrossRef]
- Revesz, L.; Schlapbach, A.; Aichholz, R.; Dawson, J.; Feifel, R.; Hawtin, S.; Littlewood-Evans, A.; Koch, G.; Kroemer, M.; Möbitz, H.; et al. In vivo and in vitro SAR of tetracyclic MAPKAP-K2 (MK2) inhibitors. Part II. Bioorg. Med. Chem. Lett. 2010, 20, 4719–4723. [Google Scholar] [CrossRef]
- Bose, G.; Bracht, K.; Bednarski, P.J.; Lalk, M.; Langer, P. Synthesis, reactions and structure-activity relationships of 1-hydroxyspiro[2.5]cyclooct-4-en-3-ones: Illudin analogs with in vitro cytotoxic activity. Bioorg. Med. Chem. 2006, 14, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Nord, C.; Menkis, A.; Broberg, A. Cytotoxic Illudane Sesquiterpenes from the Fungus Granulobasidium vellereum (Ellis and Cragin) Jülich. J. Nat. Prod. 2015, 78, 2559–2564. [Google Scholar] [CrossRef]
- Richter, R.; Von Reuß, S.H.; König, W.A. Spirocyclopropane-type sesquiterpene hydrocarbons from Schinus terebinthifolius Raddi. Phytochemistry 2010, 71, 1371–1374. [Google Scholar] [CrossRef]
- Xiao, H.X.; Yan, Q.X.; He, Z.H.; Zou, Z.B.; Le, Q.Q.; Chen, T.T.; Cai, B.; Yang, X.W.; Luo, S.L. Total synthesis and anti-inflammatory bioactivity of (−)-majusculoic acid and its derivatives. Mar. Drugs 2021, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zou, G.; Gao, Y.; Yao, Y.; Peng, C.; Shu, J.; Yang, M. A new triterpenoid and a new flavonoid glycoside isolated from Bupleurum marginatum and their anti-inflammatory activity. Nat. Prod. Res. 2020, 34, 3492–3498. [Google Scholar] [CrossRef] [PubMed]
- Köllmann, C.; Wiechert, S.M.; Jones, P.G.; Pietschmann, T.; Werz, D.B. Synthesis of 4′/5′-Spirocyclopropanated Uridine and d -Xylouridine Derivatives and Their Activity against the Human Respiratory Syncytial Virus. Org. Lett. 2019, 21, 6966–6971. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Z.; He, H.; Xiao, F.; Lin, F.; Sun, Y.; Hou, L.; Shen, L.; Han, L.; Zeng, M.; et al. Discovery of 3,3′-Spiro[Azetidine]-2-oxo-indoline Derivatives as Fusion Inhibitors for Treatment of RSV Infection. ACS Med. Chem. Lett. 2018, 9, 94–97. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Reddy, G.C.; Kovela, S.; Relitti, N.; Urabe, V.K.; Prichard, B.E.; Jurica, M.S. Enantioselective Synthesis of a Cyclopropane Derivative of Spliceostatin A and Evaluation of Bioactivity. Org. Lett. 2018, 20, 7293–7297. [Google Scholar] [CrossRef]
- Laroche, C.; Behr, J.-B.; Szymoniak, J.; Bertus, P.; Schütz, C.; Vogel, P.; Plantier-Royon, R. Spirocyclopropyl pyrrolidines as a new series of alpha-L-fucosidase inhibitors. Bioorg. Med. Chem. 2006, 14, 4047–4054. [Google Scholar] [CrossRef]
- Sandanayaka, V.P.; Prashad, A.S.; Yang, Y.; Williamson, R.T.; Lin, Y.I.; Mansour, T.S. Spirocyclopropyl beta-lactams as mechanism-based inhibitors of serine beta-lactamases. Synthesis by rhodium-catalyzed cyclopropanation of 6-diazopenicillanate sulfone. J. Med. Chem. 2003, 46, 2569–2571. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Kuhen, K.L.; Anaclerio, B.; Wu, B.; Wolff, K.; Yin, H.; Bursulaya, B.; Caldwell, J.; Karanewsky, D.; He, Y. Design, synthesis, and biological evaluations of novel quinolones as HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 4246–4251. [Google Scholar] [CrossRef]
- Link, J.O.; Taylor, J.G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.; Kirschberg, T.; Sun, J.; Squires, N.; et al. Discovery of ledipasvir (GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014, 57, 2033–2046. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagina, A.N.; Tretyakova, E.O.; Bushmarinov, I.S.; Nikishin, G.I. Stereoselective Electrocatalytic Cyclization of 4,4′-(Arylmethylene)bis(1H-pyrazol -5-ols) to (5R*,6R*)-11-Aryl-4,10-dimethyl-2,8-diphenyl-2,3,8,9-tetraazadispiro[4.0.4.1]undeca-3,9-diene-1,7-diones. Synthesis 2011, 3015–3019. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeeva, E.O.; Vereshchagin, A.N.; Nasybullin, R.F.; Egorov, M.P. Electrocatalytic stereoselective transformation of aldehydes and two molecules of pyrazolin-5-one into (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes. Catal. Sci. Technol. 2015, 5, 2384–2387. [Google Scholar] [CrossRef]
- Itokawa, M.; Miyata, T.; Arai, M. Detection and Treatment of Schizophrenia. U.S. Patent 8,809,329 B2, 19 August 2014. [Google Scholar]
- Rodríguez-Gutiérrez, S.V.; Barreiro-Costa, O.; León, C.D.A.; Heredia-Moya, J. Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones. Chem. Proc. 2021, 3, 8356. [Google Scholar] [CrossRef]
- Teran, R.; Guevara, R.; Mora, J.; Dobronski, L.; Barreiro-Costa, O.; Beske, T.; Pérez-Barrera, J.; Araya-Maturana, R.; Rojas-Silva, P.; Poveda, A.; et al. Characterization of Antimicrobial, Antioxidant, and Leishmanicidal Activities of Schiff Base Derivatives of 4-Aminoantipyrine. Molecules 2019, 24, 2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi-Jaberi, Z.; Shams, M.M.R.; Pooladian, B. Expeditious, four-component synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives catalyzed by trichloroacetic acid or ceric sulfate. Acta Chim. Slov. 2013, 60, 105–108. [Google Scholar]
- Jin, T.; Zhao, R.; Li, T. An one-pot three-component process for the synthesis of. Int. J. Mol. Sci. 2006, 176–182. [Google Scholar]
- Dorofeeva, E.; Elinson, M.; Vereshchagin, A.; Nigmatov, A.; Bushmarinov, I.; Nikishin, G. Stereoselective Thermal Isomerization of Bis(spiropyrazolone)cyclopropanes into (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolones. Synlett 2013, 24, 827–830. [Google Scholar] [CrossRef]
- Xiaoliu, L.; Daming, D.; Yongmei, W.; Jiben, M. Solid state reactions of nitrogenous heterocyclic compounds (I)—Solid state reactions of 3-methyl-1-phenyl-5-pyrazolone with carbonyl compounds. Sci. China Ser. B Chem. 1997, 40, 205–214. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M. den Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- World Health Organization. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis; Technical Report of the TDR Disease Reference Group on Chagas Disease, Human African Trypanosomiasis and Leishmaniasis; WHO Technical Report Series; WHO: Geneva, Switzerland, 2012; Volume 975, pp. 26–29. [Google Scholar]
- World Health Organization. Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; WHO Press: Geneve, Switzerland, 2010; Volume 949, pp. 54–73. [Google Scholar]
- Uzcanga, G.; Lara, E.; Gutiérrez, F.; Beaty, D.; Beske, T.; Teran, R.; Navarro, J.-C.; Pasero, P.; Benitez, W.; Poveda, A. Nuclear DNA replication and repair in parasites of the genus Leishmania: Exploiting differences to develop innovative therapeutic approaches. Crit. Rev. Microbiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy--challenges and opportunities. Clin. Microbiol. Infect. 2011, 17, 1478–1483. [Google Scholar] [CrossRef] [Green Version]
- Stirke, A.; Celiesiute-Germaniene, R.; Zimkus, A.; Zurauskiene, N.; Simonis, P.; Dervinis, A.; Ramanavicius, A.; Balevicius, S. The link between yeast cell wall porosity and plasma membrane permeability after PEF treatment. Sci. Rep. 2019, 9, 14731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouida, M.; Tounekti, O.; Belhadj, O.; Mir, L.M. Comparative Roles of the Cell Wall and Cell Membrane in Limiting Uptake of Xenobiotic Molecules by Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2003, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Mahto, K.K.; Prasad, R. Lipidomics and in vitro azole resistance in Candida albicans. OMICS 2013, 17, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeva, V.; Galutzov, B.; Teissie, J. Evidence that Pulsed Electric Field Treatment Enhances the Cell Wall Porosity of Yeast Cells. Appl. Biochem. Biotechnol. 2014, 172, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cantero, A.; Thomson, P.; Paredes, K.; Guarro, J.; Capilla, J. Antifungal susceptibility of Saccharomyces cerevisiae and therapy in a murine model of disseminated infection. Rev. Iberoam. Micol. 2019, 36, 37–40. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Dhawan, A.; Bajpayee, M.; Parmar, D. Comet assay: A reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol. 2009, 25, 5–32. [Google Scholar] [CrossRef]
- Mahajan, P.S.; Nikam, M.D.; Khedkar, V.; Jha, P.; Badadhe, P.V.; Gilla, C.H. An Organocatalyzed Efficient One-pot Synthesis, Biological Evaluation, and Molecular Docking Studies of 4,4′-(Arylmethylene)bis-(3-methyl-1- phenyl-1H-pyrazol-5-ols). J. Heterocycl. Chem. 2017, 54, 1109–1120. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Ayazi-Nasrabadi, R.; Baghery, S. Synthesis and characterization of two novel biological-based nano organo solid acids with urea moiety and their catalytic applications in the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol), coumarin-3-carboxylic acid and cinnamic ac. RSC Adv. 2015, 5, 71942–71954. [Google Scholar] [CrossRef]
- Shirini, F.; Seddighi, M.; Mazloumi, M.; Makhsous, M.; Abedini, M. One-pot synthesis of 4,4 -(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) catalyzed by Brönsted acidic ionic liquid supported on nanoporous Na+-montmorillonite. J. Mol. Liq. 2015, 208, 291–297. [Google Scholar] [CrossRef]
- Frankowski, K.J.; Liu, R.; Milligan, G.L.; Moeller, K.D.; Aubé, J. Practical Electrochemical Anodic Oxidation of Polycyclic Lactams for Late Stage Functionalization. Angew. Chem. Int. Ed. 2015, 54, 10555–10558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Pang, L.; Wang111, A. Synthesis of 4,4′-Arylmethylene-bis(3-methyl-5-pyrazolones) using Diammonium Hydrogen Phosphate as an Efficient and Versatile Catalyst in Aqueous Media. Asian J. Chem. 2011, 23, 749–751. [Google Scholar]
- Romero-Benavides, J.C.; Ortega-Torres, G.C.; Villacis, J.; Vivanco-Jaramillo, S.L.; Galarza-Urgilés, K.I.; Bailon-Moscoso, N. Phytochemical Study and Evaluation of the Cytotoxic Properties of Methanolic Extract from Baccharis obtusifolia. Int. J. Med. Chem. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.; Johansson, B. Quantitative DNA Damage and Repair Measurement with the Yeast Comet Assay. In DNA Repair Protocols; Bjergbæk, L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 920, pp. 101–109. [Google Scholar] [CrossRef]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.; Valero, M.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Nikzad, S.; Baradaran-Ghahfarokhi, M.; Nasri, P. Dose-response modeling using MTT assay: A short review. Life Sci. J. 2014, 11, 432–437. [Google Scholar] [CrossRef]
- Foulet, F.; Botterel, F.; Buffet, P.; Morizot, G.; Rivollet, D.; Deniau, M.; Pratlong, F.; Costa, J.M.; Bretagne, S. Detection and identification of Leishmania species from clinical specimens by using a real-time PCR assay and sequencing of the cytochrome b gene. J. Clin. Microbiol. 2007, 45, 2110–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Entry | Aldehyde | Yield 2 (%) | Yield 4 (%) |
|---|---|---|---|
| 1 | 3a | 98 | ---- a |
| 2 | 3b | 97 | 81 |
| 3 | 3c | 95 | 70 |
| 4 | 3d | 95 b | 91 |
| 5 | 3e | 92 | 87 |
| 6 | 3f | 95 | 77 |
| 7 | 3g | 84 d | 60 c |
| 8 | 3h | 93 | ---- a |
| 9 | 3i | 94 e | ---- a |
| 10 | 3j | 91 d | ---- a |
| 11 | 3k | 97 | 54 |
| 12 | 3l | 98 d | ---- a |
| 13 | 3m | 97 e | ---- a |
| 14 | 3n | 93 e | ---- a |
| 15 | 3o | 97 d | ---- a |
| 16 | 3p | 87 | 47 |
| 17 | 3q | Quant. | 85 |
| 18 | 3r | 96 | 87 |
| 19 | 3s | Quant. | 70 |
| 20 | 3t | Quant. | 84 |
| 21 | 3u | 60 | 64 |
| Human Cancer Cell Lines | |||
|---|---|---|---|
| RKO | PC-3 | HeLa | |
| 4b | NE | NE | NE |
| 4c | NE | NE | NE |
| 4d | 93.97 ± 4.09 | 90.87 ± 2.56 | 94.79 ± 4.54 |
| 4e | NE | NE | NE |
| 4f | NE | NE | NE |
| 4k | 84.00 ± 9.46 | 83.18 ± 0.45 | 113.70 ± 6.37 |
| 4p | NE | NE | NE |
| 4q | 102.20 ± 7.80 | 108.80 ± 2.77 | 85.07 ± 10.52 |
| 4r | 60.70 ± 9.95 | 49.79 ± 5.51 | 78.72 ± 7.90 |
| 4s | 63.80 ± 6.48 | 70.27 ± 1.90 | 78.45 ± 7.08 |
| 4t | NE | NE | NE |
| 4u | NE | NE | NE |
| Dox | 0.51 ± 0.01 | 0.65 ± 0.08 | 1.23 ± 0.10 |
| Leishmanicidal Activity IC50 µM a | Cytotoxicity CC50 µM | SI b | |
|---|---|---|---|
| 4b | 0.16 ± 0.00 (d) | 3.4 ± 1.5 (cd) | 21.3 |
| 4c | 0.15 ± 0.04 (d) | 2.3 ± 0.2 (d) | 15.3 |
| 4d | 0.15 ± 0.02 (d) | 3.9 ± 1.1 (bcd) | 26.0 |
| 4e | 1.48 ± 0.25 (b) | 7.3 ± 2.0 (abd) | 4.9 |
| 4f | 2.23 ± 0.07 (a) | 12.8 ± 1.2 (a) | 5.7 |
| 4k | 0.30 ± 0.09 (cd) | 3.3 ± 1.0 (cd) | 11.0 |
| 4p | 0.57 ± 0.23 (c) | 3.5 ± 0.9 (cd) | 6.1 |
| 4q | 0.28 ± 0.04 (cd) | 4.0 ± 0.8 (bcd) | 14.3 |
| 4r | 0.19 ± 0.06 (d) | 6.5 ± 0.9 (abc) | 34.2 |
| 4s | 0.19 ± 0.07 (d) | 8.6 ± 3.2 (ab) | 45.3 |
| 4t | 0.22 ± 0.05 (cd) | 2.7 ± 0.7 (d) | 12.3 |
| 4u | 0.42 ± 0.25 (cd) | 9.3 ± 3.4 (a) | 22.1 |
| Ampho B | 0.17 ± 0.07 (d) | >5.4 | >31.8 |
| MW | HBA | HBD | nrotb | PSA | M | T | R | I | cLogP | cLogS | DL | DS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4b | 434.50 | 6 | 0 | 3 | 65.34 | N | N | N | N | 4.97 | −4.83 | 4.34 | 0.49 |
| 4c | 479.50 | 9 | 0 | 4 | 111.16 | N | N | N | N | 4.05 | −5.29 | −0.77 | 0.32 |
| 4d | 479.50 | 9 | 0 | 4 | 111.16 | N | N | N | N | 4.05 | −5.29 | −0.77 | 0.32 |
| 4e | 464.52 | 7 | 0 | 4 | 74.57 | N | N | N | N | 4.90 | −4.84 | 4.32 | 0.47 |
| 4f | 464.52 | 7 | 0 | 4 | 74.57 | N | N | N | N | 4.90 | −4.84 | 4.32 | 0.47 |
| 4k | 479.50 | 9 | 0 | 4 | 111.16 | N | N | N | N | 4.05 | −5.29 | −0.77 | 0.32 |
| 4p | 452.49 | 6 | 0 | 3 | 65.34 | N | N | N | N | 5.07 | −5.14 | 3.00 | 0.43 |
| 4q | 492.53 | 8 | 0 | 5 | 91.64 | N | N | N | low | 4.88 | −4.97 | 1.92 | 0.33 |
| 4r | 502.50 | 6 | 0 | 4 | 65.34 | N | N | N | N | 5.82 | −5.60 | −2.85 | 0.17 |
| 4s | 518.49 | 7 | 0 | 5 | 74.57 | N | N | N | N | 6.07 | −5.85 | −3.73 | 0.15 |
| 4t | 452.49 | 6 | 0 | 3 | 65.34 | N | N | N | N | 5.07 | −5.14 | 3.00 | 0.43 |
| 4u | 480.59 | 6 | 0 | 4 | 90.64 | N | N | N | N | 5.44 | −5.67 | 4.45 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Costa, O.; Morales-Noboa, G.; Rojas-Silva, P.; Lara-Barba, E.; Santamaría-Aguirre, J.; Bailón-Moscoso, N.; Romero-Benavides, J.C.; Herrera, A.; Cueva, C.; Ron-Garrido, L.; et al. Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis. Molecules 2021, 26, 4960. https://doi.org/10.3390/molecules26164960
Barreiro-Costa O, Morales-Noboa G, Rojas-Silva P, Lara-Barba E, Santamaría-Aguirre J, Bailón-Moscoso N, Romero-Benavides JC, Herrera A, Cueva C, Ron-Garrido L, et al. Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis. Molecules. 2021; 26(16):4960. https://doi.org/10.3390/molecules26164960
Chicago/Turabian StyleBarreiro-Costa, Olalla, Gabriela Morales-Noboa, Patricio Rojas-Silva, Eliana Lara-Barba, Javier Santamaría-Aguirre, Natalia Bailón-Moscoso, Juan Carlos Romero-Benavides, Ana Herrera, Cristina Cueva, Lenin Ron-Garrido, and et al. 2021. "Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis" Molecules 26, no. 16: 4960. https://doi.org/10.3390/molecules26164960
APA StyleBarreiro-Costa, O., Morales-Noboa, G., Rojas-Silva, P., Lara-Barba, E., Santamaría-Aguirre, J., Bailón-Moscoso, N., Romero-Benavides, J. C., Herrera, A., Cueva, C., Ron-Garrido, L., Poveda, A., & Heredia-Moya, J. (2021). Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis. Molecules, 26(16), 4960. https://doi.org/10.3390/molecules26164960