A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives
Abstract
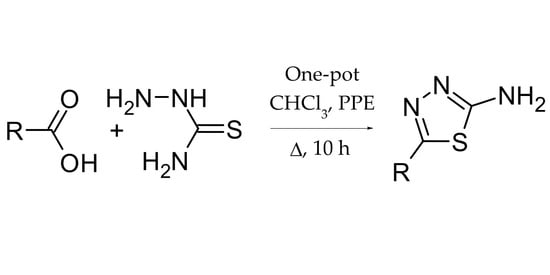
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Polkam, N.; Rayam, P.; Anireddy, J.S.; Yennam, S.; Anantaraju, H.S.; Dharmarajan, S.; Perumal, Y.; Kotapalli, S.S.; Ummanni, R.; Balasubramanian, S. Synthesis, in vitro anticancer and antimycobacterial evaluation of new 5-(2,5-dimethoxyphenyl)-1,3,4-thiadiazole-2-amino derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Wujec, M.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.; Trotsko, N.; Kuśmierz, E.; Paneth, P. Search for human DNA topoisomerase II poisons in the group of 2,5-disubstituted-1,3,4-thiadiazoles. J. Enzym. Inhib. Med. Chem. 2015, 30, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Ciftci, H.I.; Radwan, M.O.; Sever, B.; Kaplancıklı, Z.A.; Ali, T.F.S.; Koga, R.; Fujita, M.; Otsuka, M.; Özdemir, A. Design, synthesis, and biological evaluation of novel 1,3,4-thiadiazole derivatives as potential antitumor agents against chronic myelogenous leukemia: Striking effect of nitrothiazole moiety. Molecules 2017, 23, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqi, S.I.; Arshad, N.; Channar, P.A.; Perveen, F.; Saeed, A.; Larik, F.A.; Javeed, A. Synthesis, theoretical, spectroscopic and electrochemical DNA binding investigations of 1, 3, 4-thiadiazole derivatives of ibuprofen and ciprofloxacin: Cancer cell line studies. J. Photochem. Photobiol. B Biol. 2018, 189, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Er, M.; Isildak, G.; Tahtaci, H.; Karakurt, T. Novel 2-amino-1,3,4-thiadiazoles and their acyl derivatives: Synthesis, structural characterization, molecular docking studies and comparison of experimental and computational results. J. Mol. Struct. 2016, 1110, 102–113. [Google Scholar] [CrossRef]
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Dev. Ther. 2018, 12, 1545–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serban, G. Future prospects in the treatment of parasitic diseases: 2-amino-1,3,4-thiadiazoles in leishmaniasis. Molecules 2019, 24, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linciano, P.; Dawson, A.; Pöhner, I.; Costa, D.M.; Sá, M.S.; Cordeiro-Da-Silva, A.; Luciani, R.; Gul, S.; Witt, G.; Ellinger, B.; et al. Exploiting the 2-amino-1,3,4-thiadiazole scaffold to inhibit trypanosoma brucei pteridine reductase in support of early-stage drug discovery. ACS Omega 2017, 2, 5666–5683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altıntop, M.D.; Sever, B.; Özdemir, A.; Kucukoglu, K.; Onem, H.; Nadaroglu, H.; Kaplancıklı, Z.A. Potential inhibitors of human carbonic anhydrase isozymes I and II: Design, synthesis and docking studies of new 1,3,4-thiadiazole derivatives. Bioorganic Med. Chem. 2017, 25, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.T.; Rahim, F.; Taha, M.; Rehman, H.U.; Nawaz, M.; Wadood, A.; Imran, S.; Uddin, I.; Mosaddik, A.; Khan, K.M. Synthesis, in vitro α-glucosidase inhibitory potential and molecular docking study of thiadiazole analogs. Bioorganic Chem. 2018, 78, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Epishina, M.A.; Kulikov, A.S.; Ignat’Ev, N.V.; Schulte, M.; Makhova, N.N. Synthesis of 5-alkyl-2-amino-1,3,4-thiadiazoles and α,ω-bis (2-amino-1,3,4-thiadiazol-5-yl) alkanes in ionic liquids. Mendeleev Commun. 2011, 21, 331–333. [Google Scholar] [CrossRef]
- Golovlyova, S.M.; Moskvichev, Y.A.; Alov, E.M.; Kobylinsky, D.B.; Ermolaeva, V.V. Synthesis of novel five-membered nitrogen-containing heterocyclic compounds from derivatives of arylsulfonyl- and arylthioacetic and -propionic acids. Chem. Heterocycl. Compd. 2001, 37, 1102–1106. [Google Scholar] [CrossRef]
- Tsuji, T.; Takenaka, K. Convenient synthesis of 2,7-disubstituted 5h-1,3,4-thiadiazolo [3,2-a] pyrimidin-5-ones and related compounds. Bull. Chem. Soc. Jpn. 1982, 55, 637–638. [Google Scholar] [CrossRef]
- Minea, A.A. Overview of ionic liquids as candidates for new heat transfer fluids. Int. J. Thermophys. 2020, 41, 1–15. [Google Scholar] [CrossRef]
- Quistad, G.B.; Zhang, N.; Sparks, S.E.; Casida, J.E. Phosphoacetylcholinesterase: Toxicity of phosphorus oxychloride to mammals and insects that can be attributed to selective phosphorylation of acetylcholinesterase by phosphorodichloridic acid. Chem. Res. Toxicol. 2000, 13, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Shahsavari-Fard, Z. Graphite/methanesulfonic acid (GMA) as a new reagent for sulfonylation of phenols and thia-fries rearrangement of aryl sulfonates to sulfonylphenols. Helv. Chim. Acta 2005, 88, 42–52. [Google Scholar] [CrossRef]
- Kaboudin, B.; Abedi, Y. A novel synthesis of aryl mesylates via one-pot demethylation-mesylation of aryl methyl ethers using a mixture of phosphorus pentoxide in methanesulfonic acid. Synthesis 2009, 2009, 2025–2028. [Google Scholar] [CrossRef]
- Yakuschenko, I.; Pozdeeva, N.N.; Gadomsky, S.Y. A novel one-pot synthesis method of 3,4,5-triaryl-substituted 1,2,4-triazoles. Chem. Heterocycl. Compd. 2019, 55, 834–838. [Google Scholar] [CrossRef]
- Bouillot, A.M.J.; Boyer, T.; Alain Claude-Marie Daugan, A.C.-M.; Dean, A.W.; Fillmore, M.C.; Lamotte, Y. Thiadiazole Derivatives, Inhibitors of Stearoyl-CoA Desaturase. International Patent WO 2008104524 A1, 4 September 2008. [Google Scholar]
- Dixon, L.A. Polyphosphate Ester. In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd., 2001; Published Online. [Google Scholar] [CrossRef]
- Available online: https://scifinder-n.cas.org/ (accessed on 3 August 2021).
- Aly, A.A.; Hassan, A.A.; Makhlouf, M.M.; Bräse, S. Chemistry and biological activities of 1,2,4-triazolethiones—antiviral and anti-infective drugs. Molecules 2020, 25, 3036. [Google Scholar] [CrossRef] [PubMed]


| Precursor (RCOOH) | Thiadiazol 1 | Yield, % |
|---|---|---|
| Benzoic acid |  | 64.4 |
| 3-Phenylpropionic acid |  | 47.8 |
| Phenoxyacetic acid |  | 44.4 |
| 2-Naphthaleneacetic acid |  | 67.2 |
| adipic acid |  | 70.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokovina, T.S.; Gadomsky, S.Y.; Terentiev, A.A.; Sanina, N.A. A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives. Molecules 2021, 26, 5159. https://doi.org/10.3390/molecules26175159
Kokovina TS, Gadomsky SY, Terentiev AA, Sanina NA. A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives. Molecules. 2021; 26(17):5159. https://doi.org/10.3390/molecules26175159
Chicago/Turabian StyleKokovina, Tatiana S., Svyatoslav Y. Gadomsky, Alexei A. Terentiev, and Nataliya A. Sanina. 2021. "A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives" Molecules 26, no. 17: 5159. https://doi.org/10.3390/molecules26175159
APA StyleKokovina, T. S., Gadomsky, S. Y., Terentiev, A. A., & Sanina, N. A. (2021). A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives. Molecules, 26(17), 5159. https://doi.org/10.3390/molecules26175159