Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus
Abstract
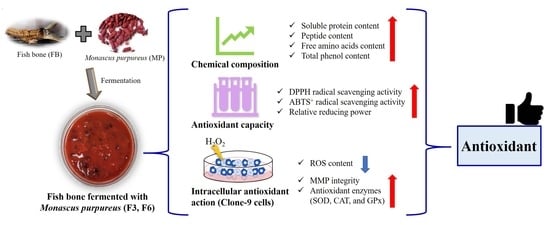
:1. Introduction
2. Results
2.1. Chemical Composition of FB, F3, and F6
2.2. Antioxidant Capacity of FB, F3, and F6
2.3. Cell Viability of FB, F3, and F6
2.4. Intracellular Antioxidant Action of FB, F3, and F6
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Sample
4.3. Chemical Composition Analysis
4.3.1. Determination of Soluble Protein Content
4.3.2. Determination of Peptides Content
4.3.3. Composition of Free Amino Acid
4.3.4. Determination of Total Phenolic Content
4.4. Antioxidant Capacity Analysis
4.4.1. DPPH Radical Scavenging Activity
4.4.2. ABTS+ Radical Scavenging Activity
4.4.3. Relative Reducing Power
4.5. Intracellular Antioxidant Action Analysis
4.5.1. Cell Culture and Treatment
4.5.2. Cell Viability Assay
4.5.3. ROS Content Assay
4.5.4. MMP Assay
4.5.5. SOD Activity Assay
4.5.6. CAT Activity Assay
4.5.7. GPx Activity Assay
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cuia, C.; Ruan, Z. Fermentation enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Stchigel, A.M.; Cano, J.F.; Abdullah, S.K.; Guarro, J. New and interesting species of Monascus from soil, with a key to the known species. Stud. Mycol. 2004, 50, 299–306. [Google Scholar]
- Li, X.M.; Shen, X.H.; Duan, Z.W.; Guo, S.R. Advances on the pharmacological effects of red yeast rice. Chin. J. Nat. Med. 2011, 9, 161–166. [Google Scholar] [CrossRef]
- Patel, S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: A review on pros and cons. World J. Microbiol. Biotechnol. 2016, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, H.; Xue, D. Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem. 2017, 226, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Suraiya, S.; Lee, J.M.; Cho, H.J.; Jang, W.J.; Kim, D.G.; Kim, Y.O.; Kong, I.S. Monascus spp. fermented brown seaweeds extracts enhance bio-functional activities. Food Biosci. 2018, 21, 90–99. [Google Scholar] [CrossRef]
- Huang, C.S.; Hu, H.H.; Tsai, Y.M.; Chang, W.T. In vitro effects of Monascus purpureus on antioxidation activity during fermentation of Kinmen sorghum liquor waste. J. Biosci. Bioeng. 2013, 115, 418–423. [Google Scholar] [CrossRef]
- Cheng, J.; Choi, B.K.; Yang, S.H.; Suh, J.W. Effect of fermentation on the antioxidant activity of rice bran by Monascus pilosus KCCM60084. J. Appl. Biol. Chem. 2016, 59, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Park, J.W. Effects of nano-scaled fish bone on the gelation properties of Alaska pollock surimi. Food Chem. 2014, 150, 463–468. [Google Scholar] [CrossRef]
- FAO. Yearbooks of Fisheries Satistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Baehaki, A.; Nopianti, R.; Anggraeni, S. Antioxidant activity of skin and bone collagen hydrolyzed from striped catfish (Pangasius pangasius) with papain enzyme. J. Chem. Pharm. Res. 2015, 7, 131–135. [Google Scholar]
- Saisavoey, T.; Sangtanoo, P.; Reamtong, O.; Karnchanatat, A. Free radical scavenging and anti-inflammatory potential of a protein hydrolysate derived from salmon bones on RAW264.7 macrophage cells. J. Sci. Food Agric. 2019, 99, 5112–5121. [Google Scholar] [CrossRef]
- Huang, M.L.H.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: Cross-talk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.H.; Kang, Y.M.; Park, J.H.; Yu, D.U.; Jeong, E.U.; Lee, M.S.; Kim, Y.M. Enhancement of polyphenol content and antioxidant activity of brown alga Eisenia bicyclis extract by microbial fermentation. Fish Aquat. Sci. 2011, 14, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N.; Rajauria, G. Effect of heating and probiotic fermentation on the phytochemical content and antioxidant potential of edible Irish brown seaweeds. Bot. Marina 2012, 55, 527–537. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, E.J.; Jung, Y.R.; Ko, H.J. The antioxidant and whitening activities of seaweeds mixture fermentation extracts. J. Soc. Cosmet. Sci. Korea 2018, 44, 327–334. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.K.; Hwang, J.W.; Han, Y.K.; Kim, S.E.; Jeong, J.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Radical scavenging activities of Undaria pinnatifida extracts fermented with Cordyceps militaris mycelia. J. Microbiol. Biotechnol. 2015, 25, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Pervin, M.; Kim, D.H.; Kim, Y.J.; Lee, J.J.; Pyo, H.J.; Lee, C.W.; Lim, B.O. DNA protection and antioxidant and anti-inflammatory activities of water extract and fermented hydrolysate of abalone (Haliotis discus hannai Ino). Food Sci. Biotechnol. 2015, 24, 689–697. [Google Scholar] [CrossRef]
- Godinho, I.; Pires, C.; Pedro, S.; Teixeira, B.; Mendes, R.; Nunes, M.L.; Batista, I. Antioxidant properties of fish protein hydrolysates prepared from cod protein hydrolysate by Bacillus sp. Appl. Biochem. Biotechnol. 2016, 178, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Vijayabaskar, P.; Shiyamala, V. Antioxidant properties of seaweed polyphenol from Turbinaria ornate (Turner) J. Agardh, 1848. Asian Pac. J. Trop. Biomed. 2012, 2, 95–98. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.C.; Kim, J.K.; Park, Y.E.; Hwang, H.S.; Kwon, G.S.; Lee, J.B. Antioxidant and whitening effects of the fermentation of barley seeds (Hordeum vulgare L.) using lactic acid bacteria. J. Life Sci. 2018, 28, 444–453. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. aurantiaca) by fermentation with kombucha ‘tea fungus’. Int. J. Food Sci. Technol. 2016, 51, 490–498. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mut. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bei, Q.; Wu, Y.; Liao, W.; Wu, Z. Characterization of soluble and insoluble bound polyphenols from Psidium guajava L. leaves co-fermented with Monascus anka and Bacillus sp. and their bio-activities. J. Funct. Foods 2017, 32, 149–159. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Bhatti, M.S. Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour. Effic. Technol. 2016, 2, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Shin, K.H.; Kim, S.K. Muscle protein hydrolysates and amino acid composition in fish. Mar. Drugs 2021, 19, 377. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.; Zhang, X.; Li, Y.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Chen, Z. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef]
- Mao, X.; Liu, P.; He, S.; Xie, J.; Kan, F.; Yu, C.; Li, Z.; Xue, C.; Lin, H. Antioxidant properties of bio-active substances from shrimp head fermented by Bacillus licheniformis OPL-007. Appl. Biochem. Biotechnol. 2013, 171, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yang, S.H. Isolation of Weissella strains as potent probiotics to improve antioxidant activity of salted squid by fermentation. J. Appl. Biol. Chem. 2018, 61, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Najafian, L.; Babji, A.S. Fractionation and identification of novel antioxidant peptides from fermented fish (pekasam). J. Food Meas. Charact. 2018, 12, 2174–2183. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K.; Muhammed, A.; Jeyaram, K.; Talukdar, N.C. Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J. Funct. Foods 2015, 14, 650–658. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Wen, Q.; Cao, X.; Chen, Z.; Xiong, Z.; Liu, J.; Cheng, Z.; Zheng, Z.; Long, C.; Zheng, B.; Huang, Z. An overview of Monascus fermentation processes for monacolin K production. Open Chem. 2020, 18, 10–21. [Google Scholar] [CrossRef]
- Yudiarti, T.; Sugiharto, S.; Isroli, I.; Widiastuti, E.; Wahyuni, H.I.; Sartono, T.A. Effect of fermentation using Chrysonillia crassa and Monascus purpureus on nutritional quality, antioxidant, and antimicrobial activities of used rice as a poultry feed ingredient. J. Adv. Vet. Anim. Res. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, Y.; Wang, P.; Zhao, M.; Li, L.; Hu, X.; Chen, F. Simultaneous determination of free amino acids in Pu-erh tea and their changes during fermentation. Food Chem. 2016, 194, 643–649. [Google Scholar] [CrossRef]
- Peralta, E.M.; Hatate, H.; Kawabe, D.; Kuwahara, R.; Wakamatsu, S.; Yuki, T.; Murata, H. Improving antioxidant activity and nutritional components of Philippine salt-fermented shrimp paste through prolonged fermentation. Food Chem. 2008, 111, 72–77. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bak, M.J.; Jeong, W.S.; Kim, K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.Y.; Hsieh, S.L.; Ciou, J.Y.; Huang, Y.W.; Leang, J.Y.; Chen, M.H.; Hou, C.Y. Lemon juice bioactivity in vitro increased with lactic acid fermentation. Int. J. Food Prop. 2021, 24, 28–40. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) muscle: Purification, identification, and cytoprotective function on HepG2 Cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Gowd, V.; Bao, T.; Chen, W. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res. Int. 2019, 120, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Lin, Y.T.; Lin, R.D.; Huang, W.J.; Lee, M.H. Neurocytoprotective effects of aliphatic hydroxamates from lovastatin, a secondary metabolite from Monascus-fermented red mold rice, in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. ACS Chem. Neurosci. 2015, 6, 716–724. [Google Scholar] [CrossRef]
- Fan, J.P.; Fan, C.; Dong, W.M.; Gao, B.; Yuan, W.; Gong, J.S. Free radical scavenging and anti-oxidative activities of an ethanol-soluble pigment extract prepared from fermented Zijuan Pu-erh tea. Food Chem. Toxicol. 2013, 59, 527–533. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Ramdall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.I. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Anti-oxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–344. [Google Scholar] [CrossRef]
- Oyaizu, M. Anti-oxidative activities of browning products of glucosamine fractionated by organic solvent and thin layer chromatography. Nippon. Shokuhin Kogyo Gakkaishi 1988, 35, 771–775. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]




| Sample | Soluble Protein (mg/mL) | Peptides (mg/mL) | Total Phenolic (μg/mL) |
|---|---|---|---|
| FB | 10.06 ± 0.53 c | 4.42 ± 0.22 b | 0.71 ± 0.02 c |
| F3 | 19.33 ± 0.81 b | 10.43 ± 1.85 a | 3.03 ± 0.06 a |
| F6 | 21.14 ± 0.77 a | 11.89 ± 0.45 a | 2.36 ± 0.02 b |
| Parameter | FB | F3 | F6 | |
|---|---|---|---|---|
| Essential amino acid | Histidine | 10.10 ± 0.3 b | 25.76 ± 1.5 a | 23.63 ± 2.6 a |
| Isoleucine | 0.43 ± 0.0 c | 3.11 ± 0.1 a | 0.72 ± 0.0 b | |
| Leucine | 0.96 ± 0.0 b | 6.47 ± 0.3 a | 1.10 ± 0.1 b | |
| Lysine | 2.82 ± 0.2 a | 0.21 ± 0.0 b | 3.05 ± 0.2 a | |
| Methionine | 0.68 ± 0.0 c | 5.62 ± 0.2 a | 2.68 ± 0.3 b | |
| Phenylalanine | 1.35 ± 0.1 b | 1.10 ± 0.0 c | 2.18 ± 0.1 a | |
| Threonine | 0.93 ± 0.0 c | 1.44 ± 0.0 b | 1.80 ± 0.2 a | |
| Tryptophan | 0.11 ± 0.0 b | 0.20 ± 0.0 b | 1.03 ± 0.1 a | |
| Valine | 0.88 ± 0.1 b | 5.82 ± 0.1 a | 0.46 ± 0.0 c | |
| Total | 18.26 ± 3.1 c | 49.74 ± 8.0 a | 36.64 ± 7.4 b | |
| Non-essential amino acid | Alanine | 1.87 ± 0.0 c | 11.07 ± 0.1 a | 2.15 ± 0.1 b |
| Arginine | 0.64 ± 0.0 b | 0.44 ± 0.0 c | 1.47 ± 0.2 a | |
| Asparagine | 0.04 ± 0.0 c | 0.95 ± 0.0 b | 1.76 ± 0.0 a | |
| Aspartic acid | 0.06 ± 0.0 c | 1.16 ± 0.1 b | 1.69 ± 0.1 a | |
| Cystine | 0.13 ± 0.0 b | 0.18 ± 0.0 a | 0.14 ± 0.0 a b | |
| Glutamic acid | 1.80 ± 0.1 c | 5.73 ± 0.3 a | 3.28 ± 0.1 b | |
| Glutamine | 0.03 ± 0.0 c | 0.21 ± 0.0 b | 0.27 ± 0.0 a | |
| Glycine | 2.33 ± 0.0 b | 15.32 ± 2.2 a | 2.55 ± 0.0 b | |
| Proline | 0.55 ± 0.1 c | 1.19 ± 0.1 b | 1.30 ± 0.0 a | |
| Serine | 0.26 ± 0.0 c | 2.29 ± 0.1 a | 1.82 ± 0.1 b | |
| Tyrosine | 0.79 ± 0.0 b | 0.14 ± 0.0 c | 1.60 ± 0.0 a | |
| Total | 8.51 ± 0.8 c | 38.70 ± 5.1 a | 18.02 ± 0.9 b | |
| Total free amino acids | 26.76 ± 2.2 c | 88.44 ± 6.5 a | 54.66 ± 5.0 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Hsieh, S.-L.; Gao, W.-S.; Yin, L.-J.; Dong, C.-D.; Chen, C.-W.; Singhania, R.-R.; Hsieh, S.; Chen, S.-J. Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus. Molecules 2021, 26, 5288. https://doi.org/10.3390/molecules26175288
Chen Y-T, Hsieh S-L, Gao W-S, Yin L-J, Dong C-D, Chen C-W, Singhania R-R, Hsieh S, Chen S-J. Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus. Molecules. 2021; 26(17):5288. https://doi.org/10.3390/molecules26175288
Chicago/Turabian StyleChen, Ya-Ting, Shu-Ling Hsieh, Wei-Siang Gao, Li-Jung Yin, Cheng-Di Dong, Chiu-Wen Chen, Reeta-Rani Singhania, Shuchen Hsieh, and Shu-Jen Chen. 2021. "Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus" Molecules 26, no. 17: 5288. https://doi.org/10.3390/molecules26175288
APA StyleChen, Y. -T., Hsieh, S. -L., Gao, W. -S., Yin, L. -J., Dong, C. -D., Chen, C. -W., Singhania, R. -R., Hsieh, S., & Chen, S. -J. (2021). Evaluation of Chemical Compositions, Antioxidant Capacity and Intracellular Antioxidant Action in Fish Bone Fermented with Monascus purpureus. Molecules, 26(17), 5288. https://doi.org/10.3390/molecules26175288