The Effect of Betulin Diphosphate in Wound Dressings of Bacterial Cellulose-ZnO NPs on Platelet Aggregation and the Activity of Oxidoreductases Regulated by NAD(P)+/NAD(P)H-Balance in Burns on Rats
Abstract
:1. Introduction
2. Results
2.1. Study of Microhemocirculation in a Burn Wound
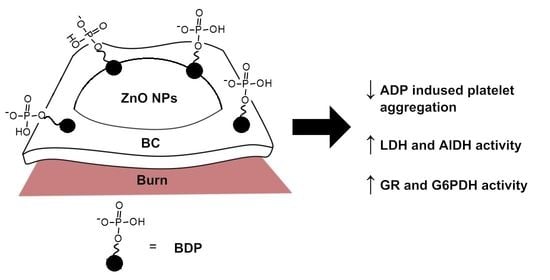
2.2. Study of Platelet Aggregation under Action of BDP
2.3. Activity of Oxidoreductases
2.3.1. LDH and AlDH-Specific Activities Controlled by Pairs of Coenzymes NAD+/NADH in Burns during Treatment with Wound Dressings Based on Bacterial Cellulose
2.3.2. The Study of the Activity of Glutathione Reductase and Glucose-6-Phosphate Dehydrogenase Catalyzed by a Pair of NADP+/NADPH Coenzymes
3. Discussion
4. Materials and Methods
4.1. Preparation of BC
4.2. Betulin-3,28-diphosphate
4.3. Zinc Oxide Nanoparticles
4.4. Oleogel ZnO NPs-BDP
4.5. Preparation of BC-ZnO NPs-BDP Composites and Their Properties
4.6. FTIR Analysis
4.7. UV Analysis
4.8. RP-HPLC Analysis
4.9. Specific Surface Areas Analysis
4.10. Chemical Composition of BC
4.11. Surface Charge and Dynamic Light Scattering Measurements
4.12. Biological Activity
4.12.1. Modeling of Thermal Burns in Animals
4.12.2. Microcirculation Research
4.12.3. Biological Analysis In Vitro
4.12.4. Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP) Preparation
4.12.5. Platelet Aggregation Measurement
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox. Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, D.C.; Rinehart, A.; Asimakis, G. Temporal changes in cellular energy following burn injury. Burns 2005, 31, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, R.; Yan, L.J. Roles of pyruvate, NADH, and mitochondrial momplex I in redox balance and imbalance in β cell function and dysfunction. J. Diabetes Res. 2015, 2015, 512618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Petriti, B.; Williams, P.A.; Lascaratos, G.; Chau, K.-Y.; Garway-Heath, D.F. Neuroprotection in glaucoma: NAD+/NADH redox state as a potential biomarker and therapeutic target. Cells 2021, 10, 1402. [Google Scholar] [CrossRef]
- Yan, L.-J. NADH/NAD+ redox imbalance and diabetic kidney disease. Biomolecules 2021, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Reinstadler, B.; Engelstad, K.; Skinner, O.S.; Stackowitz, E.; Haller, R.G.; Clish, C.B.; Pierce, K.; Walker, M.A.; Fryer, R.; et al. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J. Clin. Investig. 2021, 131, e136055. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef]
- Claridge, J.A.; Crabtree, T.D.; Pelletier, S.J.; Butler, K.; Sawyer, R.G.; Young, J.S. Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J. Trauma 2000, 48, 8–14. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [Green Version]
- Kaddoura, I.; Abu-Sittah, G.; Ibrahim, A.; Karamanoukian, R.; Papazian, N. Burn injury: Review of pathophysiology and therapeutic modalities in major burns. Ann. Burns. Fire Disasters 2017, 30, 95–102, PMID: 29021720; PMCID: PMC5627559. [Google Scholar]
- Xing, D.; Liu, L.; Marti, G.P.; Zhang, X.; Reinblatt, M.; Milner, S.M.; Harmon, J.W. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair Regen. 2011, 19, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Falamas, A.; Pinzaru, S.C.; Chis, V.; Dehelean, C. Spectroscopic investigations of newly formed betulin-cyclodextrin guest-host type complexes as potential anti skin cancer candidates. J. Mol. Struct. 2011, 993, 297–301. [Google Scholar] [CrossRef]
- Jonnalagadda, S.C.; Suman, P.; Morgan, D.C.; Seay, J.N. Recent developments on the synthesis and applications of betulin and betulinic acid derivatives as therapeutic agents. Stud. Nat. Prod. Chem. 2017, 53, 45–84. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent Achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives. Molecules 2019, 24, 3546. [Google Scholar] [CrossRef] [Green Version]
- Barret, J.P.; Podmelle, F.; Lipový, B.; Rennekampff, H.O.; Schumann, H.; Schwieger-Briel, A.; Zahn, T.R.; Metelmann, H.R. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: Results of a randomized phase III clinical trials program. Burns 2017, 43, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dong, S.; Wang, L.; Liu, Y. The effect of triterpenoid saponins on pancreatic lipase in vitro: Activity, conformation, kinetics, thermodynamics and morphology. Biochem. Eng. J. 2017, 125, 1–9. [Google Scholar] [CrossRef]
- Bureeva, S.; Andia-Pravdivy, J.; Symon, A.; Bichucher, A.; Moskaleva, V.; Popenko, V.; Shpak, A.; Shvets, V.; Kozlov, L.; Kaplun, A. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg. Med. Chem. 2007, 15, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, E.; Bebenek, E.; Kadela-Tomanek, M.; Latocha, M.; Jelsch, C.; Wenger, E.; Boryczka, S. Betulin phosphonates; Synthesis, structure, and cytotoxic activity. Molecules 2016, 21, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, A.; Carlson, R.M.; Karim, M.R.; Krasutsky, P.A. Inhibition of Epstein-Barr virus by the triterpenoid betulin diphosphate and uvaol. J. Microbiol. Biotechnol. 2004, 14, 1086–1088. [Google Scholar]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [Green Version]
- Krasutsky, P.A.; Carlson, R.M.; Karim, M.R. Triterpenes Having Antibacterial Activity. U.S. Patent US 6,689,767 B2, 10 February 2004. [Google Scholar]
- Krasutsky, P.A.; Carlson, R.M.; Karim, M.R. Triterpenes Having Human Antifungal and Antiyeast Activity. U.S. Patent US 6,642,217 B2, 4 November 2003. [Google Scholar]
- Chrobak, E.; Kadela-Tomanek, M.; Bębenek, E.; Marciniec, K.; Wietrzyk, J.; Trynda, J.; Pawełczak, B.; Kusz, J.; Kasperczyk, J.; Chodurek, E.; et al. New phosphate derivatives of betulin as anticancer agents: Synthesis, crystal structure, and molecular docking study. Bioorg. Chem. 2019, 87, 613–628. [Google Scholar] [CrossRef]
- Chrobak, E.; Jastrzębska, M.; Bębenek, E.; Kadela-Tomanek, M.; Marciniec, K.; Latocha, M.; Wrzalik, R.; Kusz, J.; Boryczka, S. Molecular structure, in vitro anticancer study and molecular docking of new phosphate derivatives of betulin. Molecules 2021, 26, 737. [Google Scholar] [CrossRef]
- Orchel, A.; Chodurek, E.; Jaworska-Kik, M.; Paduszyński, P.; Kaps, A.; Chrobak, E.; Bębenek, E.; Boryczka, S.; Borkowska, P.; Kasperczyk, J. Anticancer activity of the acetylenic derivative of betulin phosphate involves induction of necrotic-like death in breast cancer cells in vitro. Molecules 2021, 26, 615. [Google Scholar] [CrossRef]
- Melnikova, N.; Balakireva, A.; Orekhov, D.; Kamorin, D.; Didenko, N.; Malygina, D.; Knyazev, A.; Novopoltsev, D.; Solovyeva, A. Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns. Pharmaceuticals 2021, 14, 492. [Google Scholar] [CrossRef]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound healing composite materials of bacterial cellulose and zinc oxide nanoparticles with immobilized betulin diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for drug delivery and theranostic applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Wasim, M.; Khan, M.R.; Mushtaq, M.; Naeem, A.; Han, M.; Wei, Q. Surface modification of bacterial cellulose by copper and zinc oxide sputter coating for UV-resistance/antistatic/antibacterial characteristics. Coatings 2020, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, J.; Lin, H.; Ren, X.; Tian, H.; Liang, Y.; Wang, W.; Wang, Y.; Yin, M.; Huang, Y.; et al. In situ fabrication of nano ZnO/BCM biocomposite based on MA modified bacterial cellulose membrane for antibacterial and wound healing. Int. J. Nanomed. 2020, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole derivative-capped gold nanoparticles as an effective bactericide in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef]
- Danciu, C.; Pinzaru, I.; Coricovac, D.; Andrica, F.; Sizemore, I.; Dehelean, C.; Baderca, F.; Lazureanu, V.; Soica, C.; Mioc, M.; et al. Betulin silver nanoparticles qualify as efficient antimelanoma agents in in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2019, 134, 1–19. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The cytotoxic effects of betulin-conjugated gold nanoparticles as stable formulations in normal and melanoma cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Aritonang, H.F.; Kamea, O.E.; Koleangan, H.; Wuntu, A.D. Biotemplated synthesis of Ag-ZnO nanoparticles/bacterial cellulose nanocomposites for photocatalysis application. Polym.-Plast. Technol. Mater. 2020, 59, 1292–1299. [Google Scholar] [CrossRef]
- Aragão, G.F.; Carneiro, L.M.V.; Júnior, A.P.F.; Bandeira, P.N.; Lemos, T.L.G.; Viana, G.S.B. Antiplatelet activity of α.- and β.-amyrin, isomeric mixture from protium heptaphyllum. Pharm. Biol. 2007, 45, 343–349. [Google Scholar] [CrossRef]
- Babalola, I.T.; Shode, F.; Adelakun, E.; Opoku, A.; Mosa, R. Platelet-aggregation inhibitory activity of oleanolic acid, ursolic acid, betulinic acid, and maslinic acid. J. Pharmacogn. Phytochem. 2013, 1, 54–60. [Google Scholar]
- Osunsanmi, F.O.; Zaharare, G.E.; Oyinloye, B.E.; Mosa, R.A.; Ikhile, M.I.; Shode, F.O.; Ogunyinka, I.B.; Opoku, A.R. Antithrombotic, anticoagulant and antiplatelet activity of betulinic acid and 3β-acetoxybetulinic acid from Melaleuca bracteata ‘Revolution Gold’ (Myrtaceae) Muell leaf. Trop. J. Pharm. Res. 2018, 17, 1983–1989. [Google Scholar] [CrossRef] [Green Version]
- Habila, A.J.; Habila, J.D.; Shode, F.O.; Opoku, A.R.; Atawodi, S.E.; Umar, I.A. Inhibitory effect of betulinic acid and 3-acetoxybetulinic acid on rat platelet aggregation. Afr. J. Pharmacy Pharmacol. 2013, 7, 2881–2886. [Google Scholar] [CrossRef]
- Tzakos, A.G.; Kontogianni, V.G.; Tsoumani, M.; Kyriakou, E.; Hwa, J.; Rodrigues, F.A.; Tselepis, A.D. Exploration of the antiplatelet activity profile of betulinic acid on human platelets. J. Agric. Food Chem. 2012, 60, 6977–6983. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Chen, T.M.; Yang, T.S.; Wang, D.S.; Lin, S.Z. Regional skin blood flow in deep burn wounds: A preliminary report. Burns 1995, 21, 340–343. [Google Scholar] [CrossRef]
- Krupatkin, A.I.; Sidorov, V.V. Functional Diagnostics of the State of Microcirculatory-Tissue Systems: Oscillations, Information, Nonlinearity: A Guide for Physicians; LIBROKOM Book House: Moscow, Russia, 2013; 496p. [Google Scholar]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Katz, A.; Sahlin, K. Regulation of lactic acid production during exercise. J. Appl. Physiol. 1988, 65, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman and company: New York, NY, USA, 2008; 1100p. [Google Scholar]
- Chattopadhyay, R.R.; Pal, M.K.; Sarkar, S. Anticoagulation and toxicity studies with phytic acid. Pharm. Pharmacol. Commun. 1995, 1, 311–313. [Google Scholar] [CrossRef]
- Brehm, M.A.; Klemm, U.; Rehbach, C.; Erdmann, N.; Kolšek, K.; Lin, H.; Aponte-Santamaría, C.; Gräter, F.; Rauch, B.H.; Riley, A.M.; et al. Inositol hexakisphosphate increases the size of platelet aggregates. Biochem. Pharmacol. 2019, 161, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Druzijanic, A.; Druzijanic, N. Inositol hexaphosphate (IP6) and colon cancer: From concepts and first experiments to clinical application. Molecules 2020, 25, 5931. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyas’kina, E.V.; Sapunova, N.B.; Bogatyreva, A.O. Isolation and characterization of the strains producing bacterial cellulose. Microbiology 2020, 14, 86–95. [Google Scholar] [CrossRef]
- Melnikova, N.B.; Malygina, D.S.; Klabukova, I.N.; Belov, D.V.; Vasin, V.A.; Petrov, P.S.; Knyazev, A.V.; Markin, A.V. Betulin-3,28-diphosphate. Physico-chemical properties and in vitro biological activity experiments. Molecules 2018, 23, 1175. [Google Scholar] [CrossRef] [Green Version]
- Bera, D.; Qian, L.; Sabui, S.; Santra, A.; Holloway, P.H. Photoluminescence of ZnO quantum dots produced by a sol-gel process. Opt. Mater. 2008, 30, 1233–1239. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Ding, N.; Ding, W.; Wang, L.; Yu, M.; Zhang, Q. Fast synthesize ZnO quantum dots via ultrasonic method. Ultrason. Sonochem. 2015, 30, 103–112. [Google Scholar] [CrossRef]
- Melnikova, N.; Vorobyova, O.; Balakireva, A.; Malygina, D.; Solovyeva, A.; Belyaeva, K.; Orekhov, D.; Knyazev, A. The new pharmaceutical compositions of zinc oxide nanoparticles and triterpenoids for the burn treatment. Pharmaceuticals 2020, 13, 207. [Google Scholar] [CrossRef]
- Martusevich, A.K.; Larionova, K.D.; Peretyagin, S.P.; Peretyagin, P.V.; Davyduk, A.V. Experimental estimation of pharmacological compositions effect on microcirculation state at early postburn period. Fundam. Res. 2013, 3, 332–336. [Google Scholar]
- Dahmus, J.D.; Bruning, R.S.; Kenney, W.L.; Alexander, L.M. Oral clopidogrel improves cutaneuos microvascular function through EDHF-dependent mechanisms in middle-aged humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R452–R458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibgatullina, G.V.; Khartendinova, L.R.; Gumerova, E.A.; Akulov, A.N.; Kostyukova, Y.A.; Nikonorova, N.A.; Rumyantseva, N.I. Methods for Determining the Redox Status of Cultured Plant Cells; Kazan (Privolzhsky) Federal University: Kazan, Russia, 2011; pp. 18–20. [Google Scholar]
- Kochetov, G.A. Practical Guide to Enzymology, 2nd ed.; Severin, S.E., Ed.; High School: Moscow, Russia, 1980; p. 272. [Google Scholar]
- Solov’eva, A.G.; Zimin, Y.V. A new way to assess the dynamics of blood metabolism in patients with thermal trauma. Mod. Technol. Med. 2012, 2, 116–117. [Google Scholar]
- Guru, S.C.; Shetty, K.T. Methodological aspects of aldehyde dehydrogenase assay by spectrophotometric technique. Alcohol 1990, 7, 397–401. [Google Scholar] [CrossRef]
- Dawson, J.M.; Heatlic, P.L. Lowry method of protein quantification evidence for photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
- Tran, N.T.; Akkawat, B.; Morales, N.P.; Rojnuckarin, P.; Luechapudiporn, R. Antiplatelet activity of deferiprone through cyclooxygenase-1 inhibition. Platelets 2019, 31, 505–512. [Google Scholar] [CrossRef]





| System | Microhemocirculation Properties | |||
|---|---|---|---|---|
| R/E Ratio | R/N Ratio | R/C Ratio | MI, Perf. un. | |
| Burn (Day 0) | 0.79 | 0.52 | 2.73 | 6.31 ± 0.57 |
| Burn (Day 10) | 0.12 | 0.20 | 1.14 | 11.94 ± 0.71 |
| Oleogel ZnO-BDP | 6.00 | 3.43 | 6.86 | 13.19 ± 1.20 |
| BC-ZnO | 2.88 | 1.92 | 6.57 | 12.96 ± 1.97 |
| BC-ZnO-BDP | 18.67 | 16.0 | 3.86 | 14.86 ± 1.35 |
| BC-ZnO-BDP (Day 21) | 17.58 | 22.57 | 7.18 | 13.26 ± 1.21 |
| Intact | 18.25 | 24.33 | 5.62 | 13.58 ± 1.62 |
| Agent | I Stage (Disaggregation–Aggregation) | II Stage (Disaggregation–Aggregation) | ||
|---|---|---|---|---|
| % Inhibition (max) | Time (min) | % Inhibition (max) | Time (min) | |
| BDP 6.65 mM | 58 | 2.71 | 90 | 5.00 |
| ZnO NPs-BDP 6.65 mM | 29 | 2.63 | 90 | 4.62 |
| ASA 1.40 mM | 38 | 2.51 | 90 | 4.71 |
| Sample | Concentration | % Inhibition (4 min) | Max. Inhibition | |
|---|---|---|---|---|
| % Inhibition (max) | Time (min) | |||
| BDP | 4.43 mM | 73 | 90 | 5.00 |
| 2.22 mM | 9 | 85 | 3.17 | |
| 0.44 mM | 53 | 55 | 3.87 | |
| ZnO NPs (11–20 nm) | 0.01% | 0 | 1 | 4.12 |
| 0.005% | 0 | 3 | 3.98 | |
| 0.001% | 0 | 4 | 4.20 | |
| ZnO NPs-BDP | 0.01%–4.43 mM | 65 | 86 | 4.83 |
| 0.005%–2.22 mM | 31 | 83 | 4.72 | |
| 0.001%–0.44 mM | 38 | 46 | 3.56 | |
| Enzyme | τ, Day | Enzyme Activity, % of Control | |||
|---|---|---|---|---|---|
| Burnt (Untreated) | Oleogel | BC-BDP | BC-ZnO NPs-BDP | ||
| LDHdirect 1 | 3 | 66.89 ± 4.21 | 81.70 ± 5.50 | 92.15 ± 3.16 | 96.21 ± 4.40 |
| 7 | 86.54 ± 4.26 | 101.58 ± 8.50 | 102.76 ± 1.03 | 110.67 ± 1.40 | |
| 10 | 96.43 ± 6.06 | 112.59 ± 6.02 | 118.28 ± 1.17 | 126.26 ± 1.73 | |
| 21 | N/a | 111.97 ± 5.78 | 129.57 ± 2.37 | 138.25 ± 2.31 | |
| LDHreverse 2 | 3 | 57.73 ± 1.54 | 88.91 ± 3.82 | 82.34 ± 12.56 | 110.05 ± 5.99 |
| 7 | 60.26 ± 2.12 | 93.89 ± 2.86 | 96.36 ± 2.68 | 105.82 ± 2.36 | |
| 10 | 72.72 ± 2.98 | 98.82 ± 3.70 | 101.90 ± 2.23 | 108.28 ± 3.55 | |
| 21 | N/a | 96.10 ± 3.22 | 107.65 ± 4.15 | 123.71 ± 3.79 | |
| τ, Day | Burnt (Untreated) | Oleogel | BC-BDP | BC-ZnO NPs-BDP |
|---|---|---|---|---|
| 3 | 6.56 | 8.28 | 6.80 | 8.70 |
| 7 | 5.30 | 7.03 | 7.13 | 7.27 |
| 10 | 5.74 | 6.67 | 6.55 | 6.52 |
| 21 | N/a | 6.53 | 6.32 | 6.81 |
| τ, Day | AlDH Activity, % of Control 1 | |||
|---|---|---|---|---|
| Burnt (Untreated) | Oleogel | BC-BDP | BC-ZnO NPs-BDP | |
| 3 | 49.05 ± 0.38 | 140.46 ± 1.78 | 139.85 ± 2.43 | 146.88 ± 2.44 |
| 7 | 48.58 ± 0.43 | 148.57 ± 1.80 | 179.77 ± 2.64 | 279.21 ± 1.80 |
| 10 | 58.19 ± 2.64 | 138.61 ± 1.13 | 172.09 ± 2.31 | 115.00 ± 4.62 |
| 21 | N/a | 109.18 ± 3.41 | 119.54 ± 2.00 | 101.53 ± 0.97 |
| Enzyme | τ, Day | Enzyme Activity, % of Control | |||
|---|---|---|---|---|---|
| Burnt (Untreated) | Oleogel | BC-BDP | BC-ZnO NPs-BDP | ||
| GR 1 | 3 | 54.04 ± 1.07 | 78.99 ± 2.06 | 85.61 ± 3.63 | 110.35 ± 6.26 |
| 7 | 62.94 ± 1.16 | 81.61 ± 4.26 | 87.60 ± 3.56 | 90.53 ± 2.89 | |
| 10 | 70.29 ± 1.04 | 89.47 ± 2.72 | 87.49 ± 2.35 | 99.45 ± 1.45 | |
| 21 | N/a | 91.52 ± 2.29 | 88.97 ± 0.83 | 115.36 ± 2.17 | |
| G6PDH 2 | 3 | 80.24 ± 1.48 | 107.15 ± 4.14 | 97.62 ± 1.90 | 134.89 ± 2.74 |
| 7 | 93.28 ± 0.45 | 123.80 ± 2.74 | 139.77 ± 1.72 | 164.20 ± 5.14 | |
| 10 | 103.55 ± 0.95 | 125.87 ± 2.92 | 146.72 ± 2.90 | 173.19 ± 3.92 | |
| 21 | N/a | 136.40 ± 4.45 | 142.85 ± 1.45 | 191.04 ± 6.82 | |
| Medium | pH | Zeta Potential, mV | Hydrodynamic Diameter (Multimodal Mode) 1 |
|---|---|---|---|
| BDP aqueous solution | 7.44 | −7.75 ± 1.74 | 80–130; 300–400 |
| BDP in phosphate buffer | 7.20 | −14.53 ± 1.34 | 60–100; 350–450 |
| Sodium salt of phytic acid in phosphate buffer | 7.20 | −13.88 ± 3.00 | 450–800; 3000–4500; 40, 60, 130 |
| H2O | 5.80 | +15.9 ± 1.20 | N/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, N.; Malygina, D.; Balakireva, A.; Peretyagin, P.; Revin, V.; Devyataeva, A.; Malafeeva, K.; Revin, V. The Effect of Betulin Diphosphate in Wound Dressings of Bacterial Cellulose-ZnO NPs on Platelet Aggregation and the Activity of Oxidoreductases Regulated by NAD(P)+/NAD(P)H-Balance in Burns on Rats. Molecules 2021, 26, 5478. https://doi.org/10.3390/molecules26185478
Melnikova N, Malygina D, Balakireva A, Peretyagin P, Revin V, Devyataeva A, Malafeeva K, Revin V. The Effect of Betulin Diphosphate in Wound Dressings of Bacterial Cellulose-ZnO NPs on Platelet Aggregation and the Activity of Oxidoreductases Regulated by NAD(P)+/NAD(P)H-Balance in Burns on Rats. Molecules. 2021; 26(18):5478. https://doi.org/10.3390/molecules26185478
Chicago/Turabian StyleMelnikova, Nina, Darina Malygina, Alyona Balakireva, Peter Peretyagin, Vadim Revin, Anna Devyataeva, Kseniya Malafeeva, and Viktor Revin. 2021. "The Effect of Betulin Diphosphate in Wound Dressings of Bacterial Cellulose-ZnO NPs on Platelet Aggregation and the Activity of Oxidoreductases Regulated by NAD(P)+/NAD(P)H-Balance in Burns on Rats" Molecules 26, no. 18: 5478. https://doi.org/10.3390/molecules26185478
APA StyleMelnikova, N., Malygina, D., Balakireva, A., Peretyagin, P., Revin, V., Devyataeva, A., Malafeeva, K., & Revin, V. (2021). The Effect of Betulin Diphosphate in Wound Dressings of Bacterial Cellulose-ZnO NPs on Platelet Aggregation and the Activity of Oxidoreductases Regulated by NAD(P)+/NAD(P)H-Balance in Burns on Rats. Molecules, 26(18), 5478. https://doi.org/10.3390/molecules26185478