Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions
Abstract
:- A BiOI photocatalyst was successfully prepared using a solvothermal method.
- The complete photodegradation of RhB dye under solar light irradiation was achieved.
- The photodegradation reaction correlated well with the first-order kinetics model.
- The BiOI photocatalyst exhibited good structural stability and reusability after five cycles.
1. Introduction
2. Experiment
2.1. Chemicals
2.2. Fabrication of BiOI
2.3. Characterization
2.4. Photocatalytic Degradation of the Pollutants
3. Results and Discussion
3.1. Characterization of the BiOI Photocatalyst
3.2. Photodegradation Study
3.2.1. Photodegradation of Pollutants
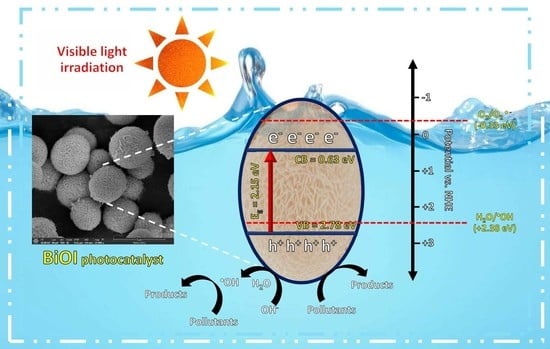
3.2.2. Photodegradation Mechanism and Cycling Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, Q.; Xin, J.; Mao, L.; Cai, X.; Ding, B.; Zhang, L.; Zhang, J.; Zheng, S.; Yang, Y. Insitu growth of BiVO4/HoVO4 heterojunction with OO bond connection for enhanced photodegradation activity. Mater. Lett. 2021, 284, 128952–128956. [Google Scholar] [CrossRef]
- Xu, J.; Bian, Z.; Xin, X.; Chen, A.; Wang, H. Size dependence of nanosheet BiVO4 with oxygen vacancies and exposed {0 0 1} facets on the photodegradation of oxytetracycline. Chem. Eng. J. 2018, 337, 684–696. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Huang, X.; Xu, N.; Li, J.; Zhou, C.; Deng, L.; He, Y. An effective strategy for boosting photoinduced charge separation of Ag3PO4 by BiVO4 with enhanced visible light photodegradation efficiency for levofloxacin and methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117986–117993. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Zhong, J.; Li, J.; Huang, S.; Ma, L. Preparation of cypress leave-like Ag2WO4/BiVO4 heterojunctions with remarkably enhanced photocatalytic activity. Mater. Lett. 2021, 283, 128793–128796. [Google Scholar] [CrossRef]
- Senasu, T.; Nanan, S. Photocatalytic performance of CdS nanomaterials for photodegradation of organic azo dyes under artificial visible light and natural solar light irradiation. J. Mater. Sci. Mater. Electron. 2017, 28, 17421–17441. [Google Scholar] [CrossRef]
- Senasu, T.; Hemavibool, K.; Nanan, S. Hydrothermally grown CdS nanoparticles for photodegradation of anionic azo dyes under UV-visible light irradiation. RSC Adv. 2018, 8, 22592–22605. [Google Scholar] [CrossRef] [Green Version]
- Kakarndee, S.; Nanan, S. SDS capped and PVA capped ZnO nanostructures with high photocatalytic performance toward photodegradation of reactive red (RR141) azo dye. J. Environ. Chem. Eng. 2018, 6, 74–94. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Watcharakitti, J.; Nanan, S. PVP-assisted synthesis of rod-like ZnO photocatalyst for photodegradation of reactive red (RR141) and Congo red (CR) azo dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 17804–17819. [Google Scholar] [CrossRef]
- Li, W.; Ding, X.; Wu, H.; Yang, H. In-situ hydrothermal synthesis of TiO2/Bi2WO6 heterojunction with enhanced photocatalytic activity. Mater. Lett. 2018, 227, 272–275. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Yang, L.; Lu, Z.; Wang, X.; Xu, G.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; et al. Controllable synthesis of Bi2WO6(001)/TiO2(001) heterostructure with enhanced photocatalytic activity. Alloys Compd. 2016, 676, 37–45. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Nanan, S. Solar light-driven photocatalyst based on bismuth molybdate (Bi4MoO9) for detoxification of anionic azo dyes in wastewater. J. Mater. Sci. Mater. Electron. 2021, 32, 1977–1991. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Visible-light-responsive photocatalyst based on ZnO/CdS nanocomposite for photodegradation of reactive red azo dye and ofloxacin antibiotic. Mater. Sci. Semicond. Process. 2020, 123, 105558. [Google Scholar] [CrossRef]
- Hassani, A.; Faraji, M.; Eghbali, P. Facile fabrication of mpg-C3N4/Ag/ZnO nanowires/Zn photocatalyst plates for photodegradation of dye pollutant. J. Photochem. Photobiol. A Chem. 2020, 400, 112665. [Google Scholar] [CrossRef]
- Arumugam, M.; Choi, M.Y. Recent progress on bismuth oxyiodide (BiOI) photocatalyst for environmental remediation. J. Ind. Eng. Chem. 2020, 81, 237–268. [Google Scholar] [CrossRef]
- Duo, F.; Fan, C.; Wang, Y.; Cao, Y.; Zhang, X. One-pot hydrothermal synthesis of a novel BiPO4/BiOBr composite with enhanced visible light photocatalytic activities. Mater. Sci. Semicond. Process. 2015, 38, 157–164. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Q.; Yan, B.; Liu, H.; Chen, K.; Wei, S.; Wu, Y.; Wang, L. Study on photocatalytic degradation of phenol by BiOI/Bi2WO6 layered heterojunction synthesized by hydrothermal method. J. Mol. Liq. 2021, 322, 114965. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Guo, J.; Zhang, J.; Wei, X.; Ma, F. Optoelectronic response and interfacial properties of BiOI/BiOX (X=F, Cl, Br) heterostructures based on DFT investigation. J. Solid State Chem. 2020, 284, 121181. [Google Scholar] [CrossRef]
- Liu, Z.S.; Ran, H.S.; Wu, B.T.; Feng, P.Z.; Zhu, Y.B. Synthesis and characterization of BiOI/BiOBr heterostructure films with enhanced visible light photocatalytic activity, Colloids Surfaces A Physicochem. Eng. Asp. 2014, 452, 109–114. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Liu, C.; Wang, D.; Yan, G.; Liu, B.; Che, G. Enhanced visible-light-induced photocatalytic degradation of tetracycline using BiOI/MIL-125(Ti) composite photocatalyst. J. Alloys Compd. 2021, 854, 157166. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Chang, M.J.; Yuan, B.; Sun, M.; Fan, S.M.; Cui, W.N.; Hui, Q.; Ni, F.R.; Li, M.Y.; et al. Fabrication of novel fibrous BiVO4/CdS heterostructures by electrospinning method for efficient visible light photodegradation. Mater. Chem. Phys. 2020, 247, 122858. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Piyavarakorn, V.; Nanan, S. Performance of solvothermally grown Bi2MoO6 photocatalyst toward degradation of organic azo dyes and fluoroquinolone antibiotics. Mater. Lett. 2020, 258, 126764–126769. [Google Scholar] [CrossRef]
- Rathi, V.; Panneerselvam, A.; Sathiyapriya, R. A novel hydrothermal induced BiVO4/g-C3N4 heterojunctions visible-light photocatalyst for effective elimination of aqueous organic pollutants. Vacuum 2020, 180, 109458–109467. [Google Scholar] [CrossRef]
- Senasu, T.; Narenuch, T.; Wannakam, K.; Chankhanittha, T.; Nanan, S. Solvothermally grown BiOCl catalyst for photodegradation of cationic dye and fluoroquinolone-based antibiotics. J. Mater. Sci. Mater. Elect. 2020, 31, 9685–9694. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Guo, H.; Liu, Y. Heterogeneous activation of peroxymonosulfate for bisphenol AF degradation with BiOI0.5Cl0.5. RSC Adv. 2019, 9, 14060–14071. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, Q.; Yang, F.; Wu, L.; Li, W.; Ren, R.; Lv, Y. Uniform flower-like BiOBr/BiOI prepared by a new method: Visible-light photocatalytic degradation, influencing factors and degradation mechanism. New J. Chem. 2019, 43, 14829–14840. [Google Scholar] [CrossRef]
- Li, S.; Lu, H.; Zhu, G.; Hojamberdiev, M.; Gao, J.; Wei, X.; Liu, P. A recyclable and stable BiOI/agarose hybrid gel photocatalyst for photodegradation of Rhodamine B. J. Mater. Sci. Mater. Elect. 2018, 29, 16454–16459. [Google Scholar] [CrossRef]
- Narenuch, T.; Senasu, T.; Chankhanittha, T.; Nanan, S. Solvothermal synthesis of CTAB capped and SDS capped BiOCl photocatalysts for degradation of rhodamine B (RhB) dye and fluoroquinolone antibiotics. J. Solid State Chem. 2020, 294, 121824. [Google Scholar] [CrossRef]
- Baral, B.; Parida, K. {040/110} Facet Isotype Heterojunctions with Monoclinic Scheelite BiVO4. Inorg. Chem. 2020, 59, 10328–10342. [Google Scholar] [CrossRef]
- Dang, M.; Tan, G.; Wang, M.; Zhang, B.; Wang, Y.; Lv, L.; Ren, H.; Xia, A. Preparation and photocatalytic enhancement mechanism of g-C3N4/(110) facet BiVO4 heterojunction induced by electrostatic field. Mater. Lett. 2021, 285, 129131–129134. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hong, S.S. Facile solvothermal synthesis of monoclinic-tetragonal heterostructured BiVO4 for photodegradation of rhodamine B. Catal. Commun. 2020, 136, 105920–105924. [Google Scholar] [CrossRef]
- Ye, S.; Zhoua, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic performance of multi-walled carbon nanotube/BiVO4 synthesized by electro-spinning process and its degradation mechanisms on oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Wang, X.; Yang, J.; Dai, Y.; He, Y.; Chen, W.; Zhang, W. Photocatalytic degradation of rhodamin B and diclofenac sodium on hollow hierarchical microspheres of BiOBr modified with sepiolite and polyvinyl pyrrolidone (PVP). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 244, 12–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Cai, W.; Zhou, J.; Li, Z. Enhanced photocatalytic performance and degradation pathway of Rhodamine B over hierarchical double-shelled zinc nickel oxide hollow sphere heterojunction. Appl. Surf. Sci. 2018, 430, 549–560. [Google Scholar] [CrossRef]
- Wermuth, T.B.; Arcaro, S.; Venturini, J.; Ribeiro, T.M.H.; de Assis Lawisch Rodriguez, A.; Machado, E.L.; de Oliveira, T.F.; de Oliveira, S.E.F.; Baibich, M.N.; Bergmann, C.P. Microwave-synthesized KNbO3 perovskites: Photocatalytic pathway on the degradation of rhodamine B. Ceram. Int. 2019, 45, 24137–24145. [Google Scholar] [CrossRef]
- Roy, J.S.; Dugas, G.; Morency, S.; Messaddeq, Y. Rapid degradation of Rhodamine B using enhanced photocatalytic activity of MoS2 nanoflowers under concentrated sunlight irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 120, 114114. [Google Scholar] [CrossRef]
- Yue, W.; Wang, Z.; Gong, J.; Wang, Z.; Dong, Y. Synthesis of tetrapod CdS by one-pot Solvothermal Method for Photodegradation to rhodamine B. Mater. Sci. Semicond. Process. 2021, 125, 105671. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narenuch, T.; Senasu, T.; Chankhanittha, T.; Nanan, S. Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions. Molecules 2021, 26, 5624. https://doi.org/10.3390/molecules26185624
Narenuch T, Senasu T, Chankhanittha T, Nanan S. Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions. Molecules. 2021; 26(18):5624. https://doi.org/10.3390/molecules26185624
Chicago/Turabian StyleNarenuch, Teerapong, Teeradech Senasu, Tammanoon Chankhanittha, and Suwat Nanan. 2021. "Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions" Molecules 26, no. 18: 5624. https://doi.org/10.3390/molecules26185624
APA StyleNarenuch, T., Senasu, T., Chankhanittha, T., & Nanan, S. (2021). Sunlight-Active BiOI Photocatalyst as an Efficient Adsorbent for the Removal of Organic Dyes and Antibiotics from Aqueous Solutions. Molecules, 26(18), 5624. https://doi.org/10.3390/molecules26185624