Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Samples
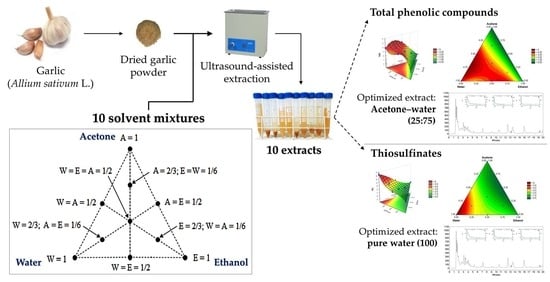
2.3. Extraction Procedure
2.4. Evaluation of Solvent Effects by Simplex Axial Design (SAD)
2.5. Total Phenolic Content (TPC)
2.6. Thiosulfinates (TS)
2.7. HPLC Analysis
2.8. Antioxidant Activities
2.8.1. Total Antioxidant Capacity (TAC)
2.8.2. DPPH Free Radical Scavenging Activity
2.8.3. Oxygen Radical Absorbance Capacity (ORAC)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Solvent Mixture Composition in TPC and TS Extraction
3.2. Mixture Designs
3.2.1. Total Phenolic Compounds (TPC)
3.2.2. Thiosulfinates (TS)
3.3. Chromatographic Profile of Garlic Optimized Extracts
3.4. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Del Valle, J.M.; Mena, C.; Budinich, M. Extraction of garlic with supercritical CO2 and conventional organic solvents. Braz. J. Chem. Eng. 2008, 25, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [Green Version]
- Samsudin, S.J.; Sufian, S.; Uemura, Y.; Herng, H.J.; Mansor, N. Quantification and characterization of allicin in garlic extract. J. Med. Bioeng. 2015, 5, 24–27. [Google Scholar] [CrossRef]
- Mamun, A.; Hasan, N.; Belal, H.; Karim, R.U.; Islam, D.; Afroz, S.; Islam, A.; Ara, T. Investigation on phytochemical content and antioxidant activity of locally grown garlic (Allium sativum L.) in Bangladesh. Int. J. Biol. Res. 2016, 1, 37–42. [Google Scholar]
- Mathialagan, R.; Mansor, N.; Shamsuddin, M.R.; Uemura, Y.; Majeed, Z. Optimisation of Ultrasonic-Assisted Extraction (UAE) of Allicin from Garlic (Allium sativum L.). Chem. Eng. Trans. 2017, 56, 1747–1752. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit—A review. Food Res. Int. 2011, 44, 1837–1842. [Google Scholar] [CrossRef]
- Abad-García, B.; Berrueta, L.A.; López-Márquez, D.M.; Crespo-Ferrer, I.; Gallo, B.; Vicente, F. Optimization and validation of a methodology based on solvent extraction and liquid chromatography for the simultaneous determination of several polyphenolic families in fruit juices. J. Chromatogr. A 2007, 1154, 87–96. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M. The use and importance of design of experiments (DOE) in process modelling in food science and technology. In Mathematical and Statistical Methods in Food Science and Technology; Granato, D., Ares, G., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–18. ISBN 9781118434635. [Google Scholar]
- Sampaio, C.R.P.; Anastácio, L.M.C.; De Francisco, T.M.G.; Ribani, R.H. Anthocyanins and phenolic compounds in five ripening stages of Byrsonima ligustrifolia after extraction optimization. J. Food Nutr. Res. 2015, 54, 365–378. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Li, Y.; Xu, S.Y. Preparation of garlic powder with high allicin content. Agric. Sci. China 2007, 6, 890–898. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Data Science Workbench, version 14. 2020. Available online: http://tibco.com (accessed on 15 August 2021).
- Strati, I.F.; Kostomitsopoulos, G.; Lytras, F.; Zoumpoulakis, P.; Proestos, C.; Sinanoglou, V.J. Optimization of polyphenol extraction from Allium ampeloprasum var. porrum through response surface methodology. Foods 2018, 7, 162. [Google Scholar] [CrossRef] [Green Version]
- Boeing, J.S.; Barizão, É.O.; Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Olech, Z.; Zaborska, W. A Spectrophotometric Assay for Total Garlic Thiosulfinates Content. Kinetic Aspects of Reaction with Chromogenic Thiols. Pol. J. Food Nutr. Sci. 2012, 62, 23–29. [Google Scholar] [CrossRef]
- Butsat, S.; Siriamornpun, S. Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C. Int. Food Res. J. 2016, 23, 180–187. [Google Scholar]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical water extraction of wild garlic ( Allium ursinum L.) and process optimization by response surface methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilić, D.P.; Nikolić, V.D.; Nikolić, L.B.; Stanković, M.Z.; Stanojević, L.P.; Cakić, M.D. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. Facta Univ.-Ser. Phys. Chem. Technol. 2011, 9, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Kiassos, E.; Mylonaki, S.; Makris, D.P.; Kefalas, P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov. Food Sci. Emerg. Technol. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Cañizares, P.; Gracia, I.; Gomez, L.A.; Martin de Argila, C.; de Rafael, L.; Garcia, A. Optimization of Allium sativum solvent extraction for the inhibition of in vitro growth of Helicobacter pylori. Biotechnol. Prog. 2002, 18, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Cañizares, P.; Gracia, I.; Gómez, L.A.; De Argila, C.M.; Boixeda, D.; García, A.; De Rafael, L. Allyl-thiosulfinates, the Bacteriostatic Compounds of Garlic against Helicobacter pylori. Biotechnol. Prog. 2004, 20, 397–401. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.H.; Cai, J.; Zhang, W.; Qi, W.L.; Wang, Z.; Liu, Z.-B.; Yang, Y. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control 2018, 86, 117–125. [Google Scholar] [CrossRef]
- Kuete, V. Allium sativum. In Medicinal Spices and Vegetables from Africa; Elsevier Academic Press: Amsterdam, The Netherlands, 2017; pp. 363–377. [Google Scholar]
- Tavares, L.; Santos, L.; Zapata Noreña, C.P. Bioactive compounds of garlic: A comprehensive review of encapsulation technologies, characterization of the encapsulated garlic compounds and their industrial applicability. Trends Food Sci. Technol. 2021, 114, 232–244. [Google Scholar] [CrossRef]
- Trifunschi, S.; Munteanu, M.F.; Pogurschi, E.; Gligor, R. Characterisation of polyphenolic compounds in Viscum album L. and Allium sativum L. extracts. Rev. Chim. 2017, 68, 1677–1680. [Google Scholar] [CrossRef]
- Chhouk, K.; Uemori, C.; Wahyudiono; Kanda, H.; Goto, M. Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chem. Eng. Process. Process Intensif. 2017, 117, 113–119. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Anwar, S.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Shari, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Alrumaihi, F. Garlic and its Active Compounds: A Novel Strategy to Fight Diseases Through Modulating Biological Activities. Pharmacogn. J. 2020, 12, 1463–1474. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Tanaka, K.; Fujita, I.; Sato, E.; Okajima, H. Antioxidant activity of thiosulfinates derived from garlic. Redox Rep. 2005, 10, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships among Bioactive Compounds Content and the Antiplatelet and Antioxidant Activities of Six Allium Vegetable Species. Food Technol. Biotechnol. 2017, 55. [Google Scholar] [CrossRef]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Guizellini, G.M.; Torres, E.A.F.D.S.; Freitas, R.A.M.S.; Saldanha, T.; Sawaya, A.C.H.F.; Gamallo, O.D.; Soares, M.J.; de Oliveira, V.S.; Sampaio, G.R. The anticholesterol oxidation effects of garlic (Allium sativum L.) and leek (Allium ampeloprasum L.) in frozen fish burgers submitted to grilling. J. Food Sci. 2020, 85, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Denre, M.; Pal, S.; Mazumdar, D.; Chakravarty, A.; Bhattacharya, A. Variation in Antioxidants and Antioxidant Activity in Garlic Cultivars. Int. J. Veg. Sci. 2013, 19, 374–383. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mlček, J.; Snopek, L.; Planetová, T. Polyphenols and antioxidant capacity in different types of garlic. Potravin. Slovak J. Food Sci. 2018, 12, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Olaleye, O.; Kolawole, O. Antimicrobial and antioxidant properties of aqueous Garlic (Allium sativum) extract against Staphylococcus aureus and Pseudomonas aeruginosa. Br. Microbiol. Res. J. Int. 2016, 14, 1–11. [Google Scholar] [CrossRef]




| Time (Min) | Water a (%) | Methanol b (%) |
|---|---|---|
| 0 | 95 | 05 |
| 12 | 85 | 15 |
| 20 | 70 | 30 |
| Run | Water | Ethanol | Acetone | TPC (mg GAE a g−1 DW b) | TS (μmol g−1 DW) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 3.82 ± 0.07 | 6.42 ± 0.22 |
| 2 | 0 | 1 | 0 | 0.84 ± 0.01 | 1.40 ± 0.09 |
| 3 | 0 | 0 | 1 | 0.35 ± 0.02 | 1.43 ± 0.18 |
| 4 | 1/2 | 0 | 1/2 | 4.28 ± 0.12 | 3.41 ± 0.15 |
| 5 | 1/2 | 1/2 | 0 | 3.62 ± 0.08 | 2.78 ± 0.25 |
| 6 | 0 | 1/2 | 1/2 | 0.26 ± 0.01 | 0.33 ± 0.13 |
| 7 | 1/3 | 1/3 | 1/3 | 3.20 ± 0.04 | 2.11 ± 0.09 |
| 8 | 1/6 | 1/6 | 2/3 | 1.71 ± 0.07 | 1.18 ± 0.16 |
| 9 | 1/6 | 2/3 | 1/6 | 2.60 ± 0.08 | 1.53 ± 0.05 |
| 10 | 2/3 | 1/6 | 1/6 | 5.84 ± 0.03 | 3.69 ± 0.31 |
| Variation Source | Sum of Squares | Degrees of Freedom | Mean Square | Calculated F-Value | Probability | |
|---|---|---|---|---|---|---|
| TPC | Model | 64.73069 | 8 | 8.091336 | 1606.606 | 0.000000 |
| Total Error | 0.10576 | 21 | 0.005036 | |||
| Lack of Fit | 0.01448 | 1 | 0.014477 | 3.172 | 0.090111 | |
| Pure Error | 0.09128 | 20 | 0.004564 | |||
| Total Adjusted | 64.83645 | 29 | 2.235740 | |||
| TS | Model | 82.58388 | 8 | 10.32299 | 268.4487 | 0.000000 |
| Total Error | 0.80754 | 21 | 0.03845 | |||
| Lack of Fit | 0.12249 | 1 | 0.12249 | 3.5761 | 0.073193 | |
| Pure Error | 0.68505 | 20 | 0.03425 | |||
| Total Adjusted | 83.39142 | 29 | 2.87557 |
| Water (%) | Ethanol (%) | Acetone (%) | TAC a (mg AAE d g−1 DW e) | DPPH IC50 b (mg mL−1) | ORAC c (µmol TE f g−1 DW) |
|---|---|---|---|---|---|
| 100 | 0 | 0 | 200.31 ± 0.93 a * | 3.40 ± 0.35 b | 882.47 ± 141.04 a |
| 75 | 0 | 25 | 171.30 ± 2.13 b | 2.88 ± 0.26 a | 794.63 ± 135.99 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Rocha, J.P.M.; Coelho, A.D.; Oliveira, A.J.M.; Mendes, L.C.; Pereira, M.M.A.; Morais, L.C.; Forim, M.R.; et al. Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.). Molecules 2021, 26, 6026. https://doi.org/10.3390/molecules26196026
Cavalcanti VP, Aazza S, Bertolucci SKV, Rocha JPM, Coelho AD, Oliveira AJM, Mendes LC, Pereira MMA, Morais LC, Forim MR, et al. Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.). Molecules. 2021; 26(19):6026. https://doi.org/10.3390/molecules26196026
Chicago/Turabian StyleCavalcanti, Vytória Piscitelli, Smail Aazza, Suzan Kelly Vilela Bertolucci, João Pedro Miranda Rocha, Adriane Duarte Coelho, Altino Júnior Mendes Oliveira, Laís Campelo Mendes, Maysa Mathias Alves Pereira, Ludmila Caproni Morais, Moacir Rossi Forim, and et al. 2021. "Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.)" Molecules 26, no. 19: 6026. https://doi.org/10.3390/molecules26196026
APA StyleCavalcanti, V. P., Aazza, S., Bertolucci, S. K. V., Rocha, J. P. M., Coelho, A. D., Oliveira, A. J. M., Mendes, L. C., Pereira, M. M. A., Morais, L. C., Forim, M. R., Pasqual, M., & Dória, J. (2021). Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.). Molecules, 26(19), 6026. https://doi.org/10.3390/molecules26196026