Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems
Abstract
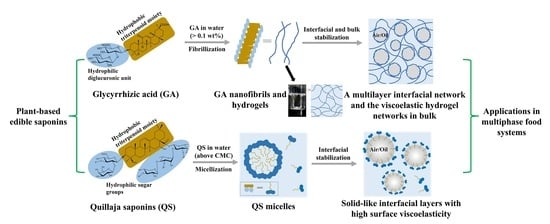
:1. Introduction
2. Biological Activities of Saponins
3. Molecular Structure and Self-Assembly in Aqueous Solutions
3.1. Saponin Micelles
3.2. Glycyrrhizic Acid Nanofibrils and Supramolecular Hydrogels
4. Behaviors of Saponin Molecules and Assemblies at Liquid Interfaces
4.1. Interfacial Properties and Configuration of Saponin Molecules
4.2. Interfacial Behaviors of Glycyrrhizic Acid Nanofibrils
5. Applications of Saponins in Colloidal Multiphase Systems
5.1. Liquid Emulsions
5.2. Gel Emulsions
5.3. Aqueous Foams
5.4. Complex Emulsion Foams
| Type of Multiphase Systems | Structural Building Blocks | Properties and Applications | Ref. |
|---|---|---|---|
| Liquid O/W emulsions | QS molecules | Properties: Nanoemulsions; high stability under pH 2–8, 0–500 mM NaCl, temperatures of 20–90 °C and after long-term storage. Applications: Delivery systems for hydrophobic bioactives; Inhibition of lipid oxidation; Controlled flavor retention and release during simulated cooking. | [29,82,83,84,85,86] |
| QS-coated nanodroplets | Properties: Multicompartment shell comprising nanodroplets; Good stability under pH values 3–7, salts 0–500 mM NaCl and temperatures 25–100 °C. Applications: Programmed release of volatiles. | [87] | |
| Gel emulsions | QS-coated nanodroplets | Properties: Stable HIPEs with 75% oil for over six months of storage. Applications: Formation of transparent oleogels (99.7% oil); Color performance | [31] |
| Aqueous foams | QS molecules | Properties: Relatively stable foams under pH values 3–5 and salt up to 500 mM NaCl; Reduced rate of Ostwald ripening. | [96] |
| QS molecules, food proteins | Properties: Improved foaming properties by proteins β-lactoglobulin and lysozyme. | [26,68,94] | |
| Complex emulsion foams | QS-coated nanodroplets | Properties: Significantly higher foamability and foam stability than QS aqueous foam. Applications: Encapsulation and controlled release of hydrophobic flavors and bioactives. | [98] |
| Type of Multiphase Systems | Structural Building Blocks | Properties and Applications | Ref. |
|---|---|---|---|
| Liquid O/W emulsions | GA molecules | Properties: Stable emulsions (pH 7.0, 0.2 μm) under pH values 5–9, salts 0–200 mM NaCl, and temperatures up to 60 °C. | [89,90] |
| GA nanofibrils | Properties: Emulsions (5 wt% oil, 0.25 wt% nanofibrils) with good stability after repeated heat treatments (80 °C, 20 min) and storage for two months. | [33] | |
| Gel emulsions | GA nanofibrils | Properties: 10–60 wt% oils; High gel strength and thixotropic recovery; Thermoresponsive properties. Applications: Oil structuring materials; Delivery vehicle for oil-soluble ingredients; Green pesticides. | [33,34,91] |
| GA nanofibrils, PGPR | Properties: W1/O/W2 gel emulsions with high yield (85.6–92.5%) and storage stability. Applications: Protection of photosensitive water-soluble cargos (Riboflavin-5′-phosphate). | [78] | |
| GA nanofibrils, SPI-pectin nanoparticles | Properties: Small droplet size, homogeneous appearance and microstructure at 1 wt% or higher nanofibril concentration. | [88] | |
| GA nanofibrils, sitosterol–oryzanol mixture | Properties: Dual-structured gel emulsions; Controlled linear and nonlinear viscoelastic behaviors. Applications: Oil structuring materials with specific textural and functional properties. | [92] | |
| GA nanofibrils, CD–MOF | Properties: Long-term stability, even under high-alkaline pH and high-temperature (70 °C). | [93] | |
| Aqueous foams | GA nanofibrils | Properties: Ultrastable foams with homogeneous appearance for at least six months at 25 °C; Without any liquid drainage; High foamability; Stimulability and processability. Applications: Controlled delivery and release; Solid template for porous materials. | [35] |
| GA nanofibrils, CNCs | Properties: Composite foams with higher elastic modulus and yield stress (especially with NaCl); Tunable stability and thermoresponsive behavior. | [79] | |
| Complex emulsion foams | GA nanofibrils | Properties: Stable emulsion foams for at least two weeks; Viscoelastic properties: Thermoresponsive behavior. | [36] |
| GA nanofibrils, CBP particles | Properties: Dual photo-/thermoresponsive emulsion foams; On-demand destabilization by multiple external stimuli. | [36] |
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, E.; Miller, R. Food Colloids: Fundamentals of Formulation; Royal Society of Chemistry: London, UK, 2001; Volume 258. [Google Scholar]
- Sagis, L.M.C. Dynamic properties of interfaces in soft matter: Experiments and theory. Rev. Mod. Phys. 2011, 83, 1367–1403. [Google Scholar] [CrossRef]
- Bos, M.A.; Van Vliet, T. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef]
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable particle-stabilized foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef] [Green Version]
- Mezzenga, R.; Schurtenberger, P.; Burbidge, A.; Michel, M. Understanding foods as soft materials. Nat. Mater. 2005, 4, 729–740. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Sagis, L.; Schroen, K. Formation, structure, and functionality of interfacial layers in food emulsions. Annu. Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.R. Functional and engineered colloids from edible materials for emerging applications in designing the food of the future. Adv. Funct. Mater. 2020, 30, 1806809. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Drusch, S. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef]
- Goral, I.; Wojciechowski, K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Thomas, R.K.; Tucker, I.; Petkov, J.T.; Stoyanov, S.D.; Denkov, N.; Golemanov, K.; Tcholakova, S.; Webster, J.R.P. Adsorption properties of plant based bio-surfactants: Insights from neutron scattering techniques. Adv. Colloid Interface Sci. 2019, 274, 102041. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja saponin characteristics and functional properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Stanimirova, R.; Marinova, K.; Tcholakova, S.; Denkov, N.D.; Stoyanov, S.; Pelan, E. Surface rheology of saponin adsorption layers. Langmuir 2011, 27, 12486–12498. [Google Scholar] [CrossRef] [PubMed]
- Golemanov, K.; Tcholakova, S.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Surface shear rheology of saponin adsorption layers. Langmuir 2012, 28, 12071–12084. [Google Scholar] [CrossRef]
- Golemanov, K.; Tcholakova, S.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Remarkably high surface visco-elasticity of adsorption layers of triterpenoid saponins. Soft Matter 2013, 9, 5738–5752. [Google Scholar] [CrossRef]
- Wojciechowski, K. Surface activity of saponin from Quillaja bark at the air/water and oil/water interfaces. Colloids Surf. B Biointerfaces 2013, 108, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Golemanov, K.; Tcholakova, S.; Denkov, N.; Pelan, E.; Stoyanov, S.D. The role of the hydrophobic phase in the unique rheological properties of saponin adsorption layers. Soft Matter 2014, 10, 7034–7044. [Google Scholar] [CrossRef] [PubMed]
- Kezwon, A.; Wojciechowski, K. Interaction of Quillaja bark saponins with food-relevant proteins. Adv. Colloid Interface Sci. 2014, 209, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Lewandowska, J.; Wojciechowski, K. Biosurfactant-protein mixtures: Quillaja Bark Saponin at water/air and water/oil interfaces in presence of beta-lactoglobulin. J. Phys. Chem. B 2012, 116, 4843–4850. [Google Scholar] [CrossRef]
- Santini, E.; Jarek, E.; Ravera, F.; Liggieri, L.; Warszynski, P.; Krzan, M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloids Surf. B Biointerfaces 2019, 181, 198–206. [Google Scholar] [CrossRef]
- Orczyk, M.; Wojciechowski, K. Comparison of the effect of two Quillaja bark saponin extracts on DPPC and DPPC/cholesterol Langmuir monolayers. Colloids Surf. B Biointerfaces 2015, 136, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocolloids 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Zhang, J.; Bing, L.; Reineccius, G.A. Comparison of modified starch and Quillaja saponins in the formation and stabilization of flavor nanoemulsions. Food Chem. 2016, 192, 53–59. [Google Scholar] [CrossRef]
- Chen, X.W.; Wang, J.M.; Guo, J.; Wan, Z.L.; Yin, S.W.; Yang, X.Q. Hierarchical high internal phase emulsions and transparent oleogels stabilized by quillaja saponin-coated nanodroplets for color performance. Food Funct. 2017, 8, 823–831. [Google Scholar] [CrossRef]
- Salminen, H.; Aulbach, S.; Leuenberger, B.H.; Tedeschi, C.; Weiss, J. Influence of surfactant composition on physical and oxidative stability of Quillaja saponin-stabilized lipid particles with encapsulated omega-3 fish oil. Colloids Surf. B Biointerfaces 2014, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.L.; Sun, Y.E.; Ma, L.L.; Guo, J.; Wang, J.M.; Yin, S.W.; Yang, X.Q. Thermoresponsive structured emulsions based on the fibrillar self-assembly of natural saponin glycyrrhizic acid. Food Funct. 2017, 8, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.L.; Sun, Y.E.; Ma, L.L.; Yang, X.Q.; Guo, J.; Yin, S.W. Responsive emulsion gels with tunable properties formed by self-assembled nanofibrils of natural saponin glycyrrhizic acid for oil structuring. J. Agric. Food Chem. 2017, 65, 2394–2405. [Google Scholar] [CrossRef]
- Ma, L.L.; Li, Q.; Du, Z.Y.; Su, E.Y.; Liu, X.; Wan, Z.L.; Yang, X.Q. A natural supramolecular saponin hydrogelator for creation of ultrastable and thermostimulable food-grade foams. Adv. Mater. Interfaces 2019, 6, 1900417. [Google Scholar] [CrossRef]
- Wan, Z.L.; Sun, Y.E.; Ma, L.L.; Zhou, F.B.; Guo, J.; Hu, S.Q.; Yang, X.Q. Long-lived and thermoresponsive emulsion foams stabilized by self-assembled saponin nanofibrils and fibrillar network. Langmuir 2018, 34, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Vinarova, L.; Vinarov, Z.; Atanasov, V.; Pantcheva, I.; Tcholakova, S.; Denkov, N.; Stoyanov, S. Lowering of cholesterol bioaccessibility and serum concentrations by saponins: In vitro and in vivo studies. Food Funct. 2015, 6, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Vinarova, L.; Vinarov, Z.; Damyanova, B.; Tcholakova, S.; Denkov, N.; Stoyanov, S. Mechanisms of cholesterol and saturated fatty acid lowering by Quillaja saponaria extract, studied by in vitro digestion model. Food Funct. 2015, 6, 1319–1330. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Trapp, M.; Marcinkowski, K.; Kobiela, T.; Geue, T. Unusual penetration of phospholipid mono- and bilayers by Quillaja bark saponin biosurfactant. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Geue, T. Complexation of phospholipids and cholesterol by triterpenic saponins in bulk and in monolayers. Biochim. Biophys. Acta Biomembr. 2016, 1858, 363–373. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Trapp, M.; Gutberlet, T. Effect of triterpene and steroid saponins on lecithin bilayers. Colloids Surf. A 2016, 510, 150–158. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Kubski, P.; Wojciechowski, K. New experimental model of pulmonary surfactant for biophysical studies. Colloids Surf. A 2017, 519, 27–33. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Paepenmuller, T.; Muller-Goymann, C.C. Influence of Quil A on liposomal membranes. Int. J. Pharm. 2014, 475, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.P.; Rivera-Patron, M.; Mourglia-Ettlin, G.; Casaravilla, C.; Yendo, A.C.A.; Fett-Neto, A.G.; Chabalgoity, J.A.; Moreno, M.; Roehe, P.M.; Silveira, F. Quillaja brasiliensis saponin-based nanoparticulate adjuvants are capable of triggering early immune responses. Sci. Rep. 2018, 8, 13582. [Google Scholar] [CrossRef] [PubMed]
- Jeepipalli, S.P.K.; Du, B.; Sabitaliyevich, U.Y.; Xu, B.J. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Sharma, P.; Tyagi, A.; Bhansali, P.; Pareek, S.; Singh, V.; Ilyas, A.; Mishra, R.; Poddar, N.K. Saponins: Extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem. Toxicol. 2021, 150, 112075. [Google Scholar] [CrossRef] [PubMed]
- Pompei, R.; Laconi, S.; Ingianni, A. Antiviral properties of glycyrrhizic acid and its semisynthetic derivatives. Mini Rev. Med. Chem. 2009, 9, 996–1001. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.F.; Wang, K.C.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; Chang, J.S. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Du, Q.H. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints 2020. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Xiao, Y.C.; Xu, L.Q.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.C.; Tan, Q.Q.; Tang, L.T.; et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Yu, S.P.; Zhu, Y.Y.; Xu, J.R.; Yao, G.T.; Zhang, P.; Wang, M.G.; Zhao, Y.F.; Lin, G.F.; Chen, H.Z.; Chen, L.L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2020, 85, 153364. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Zhang, L.M.; Wang, T.; Fu, F.H. Severe acute lung injury related to COVID-19 infection: A review and the possible role for Escin. J. Clin. Pharmacol. 2020, 60, 815–825. [Google Scholar] [CrossRef]
- Wua, C.R.; Liu, Y.; Yang, Y.Y.; Zhang, P.; Zhong, W.; Wang, Y.L.; Wang, Q.Q.; Xu, Y.; Li, M.X.; Li, X.Z.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dungan, S.R. Micellar properties of Quillaja saponin. 1. Effects of temperature, salt, and pH on solution properties. J. Agric. Food Chem. 1997, 45, 1587–1595. [Google Scholar] [CrossRef]
- Tippel, J.; Lehmann, M.; von Klitzing, R.; Drusch, S. Interfacial properties of Quillaja saponins and its use for micellisation of lutein esters. Food Chem. 2016, 212, 35–42. [Google Scholar] [CrossRef]
- Saha, A.; Adamcik, J.; Bolisetty, S.; Handschin, S.; Mezzenga, R. Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features. Angew. Chem. Int. Ed. 2015, 127, 5498–5502. [Google Scholar] [CrossRef]
- Yoshioka, H. Kinetics of the gel—sol transition of the aqueous solutions of β-glycyrrhizin studied by the temperature jump—spin probe method. J. Colloid Interface Sci. 1985, 105, 65–72. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Gao, Y.X.; Lin, Y.; Hu, J. A simple injectable moldable hydrogel assembled from natural glycyrrhizic acid with inherent antibacterial activity. ACS Appl. Bio Mater. 2019, 3, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.X.; Hu, Q. Determination of the polyacid dissociation constants of glycyrrhizic acid. Indian J. Chem. 2008, 47, 71–74. [Google Scholar]
- Matsuoka, K.; Miyajima, R.; Ishida, Y.; Karasawa, S.; Yoshimura, T. Aggregate formation of glycyrrhizic acid. Colloids Surf. A 2016, 500, 112–117. [Google Scholar] [CrossRef]
- Petrova, S.S.; Schlotgauer, A.A.; Kruppa, A.I.; Leshina, T.V. Self-association of glycyrrhizic acid. NMR study. Z. Phys. Chem. 2017, 231, 839–855. [Google Scholar] [CrossRef]
- Pagureva, N.; Tcholakova, S.; Golemanov, K.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Surface properties of adsorption layers formed from triterpenoid and steroid saponins. Colloids Surf. A 2016, 491, 18–28. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2015, 11, 91–100. [Google Scholar] [CrossRef]
- Jordens, S.; Isa, L.; Usov, I.; Mezzenga, R. Non-equilibrium nature of two-dimensional isotropic and nematic coexistence in amyloid fibrils at liquid interfaces. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isa, L.; Jung, J.M.; Mezzenga, R. Unravelling adsorption and alignment of amyloid fibrils at interfaces by probe particle tracking. Soft Matter 2011, 7, 8127–8134. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.L.; Yang, X.Q.; Sagis, L.M. Nonlinear surface dilatational rheology and foaming behavior of protein and protein fibrillar aggregates in the presence of natural surfactant. Langmuir 2016, 32, 3679–3690. [Google Scholar] [CrossRef]
- Jung, J.M.; Gunes, D.Z.; Mezzenga, R. Interfacial activity and interfacial shear rheology of native β-lactoglobulin monomers and their heat-induced fibers. Langmuir 2010, 26, 15366–15375. [Google Scholar] [CrossRef] [PubMed]
- Humblet-Hua, N.P.K.; van der Linden, E.; Sagis, L.M.C. Surface rheological properties of liquid–liquid interfaces stabilized by protein fibrillar aggregates and protein–polysaccharide complexes. Soft Matter 2013, 9, 2154–2165. [Google Scholar] [CrossRef]
- Wan, Z.L.; Yang, X.Q.; Sagis, L.M.C. Contribution of long fibrils and peptides to surface and foaming behavior of soy protein fibril system. Langmuir 2016, 32, 8092–8101. [Google Scholar] [CrossRef] [PubMed]
- Wege, H.A.; Kim, S.; Paunov, V.N.; Zhong, Q.X.; Velev, O.D. Long-term stabilization of foams and emulsions with in-situ formed microparticles from hydrophobic cellulose. Langmuir 2008, 24, 9245–9253. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Cervin, N.T.; Andersson, L.; Ng, J.B.; Olin, P.; Bergstrom, L.; Wagberg, L. Lightweight and strong cellulose materials made from aqueous foams stabilized by nanofibrillated cellulose. Biomacromolecules 2013, 14, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wan, Z.L.; Yang, X.Q. Multiple water-in-oil-in-water emulsion gels based on self-assembled saponin fibrillar network for photosensitive cargo protection. J. Agric. Food Chem. 2017, 65, 9735–9743. [Google Scholar] [CrossRef] [PubMed]
- Su, E.Y.; Li, Q.; Xu, M.Y.; Yuan, Y.; Wan, Z.L.; Yang, X.Q.; Binks, B.P. Highly stable and thermo-responsive gel foams by synergistically combining glycyrrhizic acid nanofibrils and cellulose nanocrystals. J. Colloid Interface Sci. 2021, 587, 797–809. [Google Scholar] [CrossRef]
- Ma, L.L.; Bertsch, P.; Wan, Z.L.; Yang, X.Q.; Fischer, P. Synergistic effect of glycyrrhizic acid and cellulose nanocrystals for oil-water interfacial stabilization. Food Hydrocolloids 2021, 120, 106888. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Badolato Bonisch, G.; Schafer, C.; Weiss, J. Concentration effect of Quillaja saponin—Co-surfactant mixtures on emulsifying properties. J. Colloid Interface Sci. 2018, 519, 71–80. [Google Scholar] [CrossRef]
- Uluata, S.; McClements, D.J.; Decker, E.A. Physical stability, autoxidation, and photosensitized oxidation of omega-3 oils in nanoemulsions prepared with natural and synthetic surfactants. J. Agric. Food Chem. 2015, 63, 9333–9340. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Chen, Y.J.; Wang, J.M.; Guo, J.; Yin, S.W.; Yang, X.Q. Phytosterol structured algae oil nanoemulsions and powders: Improving antioxidant and flavor properties. Food Funct. 2016, 7, 3694–3702. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.Q.; Gu, J.Y.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocolloids 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Tippel, J.; Gies, K.; Harbaum-Piayda, B.; Steffen-Heins, A.; Drusch, S. Composition of Quillaja saponin extract affects lipid oxidation in oil-in-water emulsions. Food Chem. 2017, 221, 386–394. [Google Scholar] [CrossRef]
- Doi, T.; Wang, M.Q.; McClements, D.J. Emulsion-based control of flavor release profiles: Impact of oil droplet characteristics on garlic aroma release during simulated cooking. Food Res. Int. 2019, 116, 1–11. [Google Scholar] [CrossRef]
- Chen, X.W.; Ning, X.Y.; Zou, Y.; Liu, X.; Yang, X.Q. Multicompartment emulsion droplets for programmed release of hydrophobic cargoes. Food Funct. 2019, 10, 4522–4532. [Google Scholar] [CrossRef]
- Li, Q.; He, Q.X.; Xu, M.Y.; Li, J.G.; Liu, X.; Wan, Z.L.; Yang, X.Q. Food-grade emulsions and emulsion gels prepared by soy protein-pectin complex nanoparticles and glycyrrhizic acid nanofibrils. J. Agric. Food Chem. 2020, 68, 1051–1063. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Braun, K.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Investigations into the structure-function relationship of plant-based surfactant glycyrrhizin: Interfacial behavior & emulsion formation. LWT Food Sci. Technol. 2020, 120, 108910. [Google Scholar]
- Ralla, T.; Salminen, H.; Braun, K.; Edelmann, M.; Weiss, J. Investigations into the structure-function relationship of the naturally-derived surfactant glycyrrhizin: Emulsion stability. Food Biophys. 2020, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Gao, Y.X.; Zhao, X.; Zhu, Y.Q.; Du, F.P.; Hu, J. A natural triterpene saponin-based Pickering emulsion. Chem.-Eur. J. 2018, 24, 11703–11710. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.Y.; Xie, J.; Su, E.Y.; Wan, Z.L.; Sagis, L.M.C.; Yang, X.Q. Large amplitude oscillatory shear (LAOS) for nonlinear rheological behavior of heterogeneous emulsion gels made from natural supramolecular gelators. Food Res. Int. 2021, 140, 110076. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.P.; Qin, Y.; Xu, X.M.; Jin, Z.Y. Characterization and mechanisms of novel emulsions and nanoemulsion gels stabilized by edible cyclodextrin-based metal-organic frameworks and glycyrrhizic acid. J. Agric. Food Chem. 2019, 67, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Piotrowski, M.; Popielarz, W.; Sosnowski, T.R. Short- and mid-term adsorption behaviour of Quillaja Bark Saponin and its mixtures with lysozyme. Food Hydrocolloids 2011, 25, 687–693. [Google Scholar] [CrossRef]
- Tcholakova, S.; Mustan, F.; Pagureva, N.; Golemanov, K.; Denkov, N.D.; Pelan, E.G.; Stoyanov, S.D. Role of surface properties for the kinetics of bubble Ostwald ripening in saponin-stabilized foams. Colloids Surf. A 2017, 534, 16–25. [Google Scholar] [CrossRef]
- Böttcher, S.; Scampicchio, M.; Drusch, S. Mixtures of saponins and beta-lactoglobulin differ from classical protein/surfactant-systems at the air-water interface. Colloids Surf. A 2016, 506, 765–773. [Google Scholar] [CrossRef]
- Salonen, A.; Lhermerout, R.; Rio, E.; Langevin, D.; Saint-Jalmes, A. Dual gas and oil dispersions in water: Production and stability of foamulsion. Soft Matter 2012, 8, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.W.; Yang, D.X.; Zou, Y.; Yang, X.Q. Stabilization and functionalization of aqueous foams by Quillaja saponin-coated nanodroplets. Food Res. Int. 2017, 99, 679–687. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Wan, Z.; Yang, X. Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules 2021, 26, 6075. https://doi.org/10.3390/molecules26196075
Xu M, Wan Z, Yang X. Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules. 2021; 26(19):6075. https://doi.org/10.3390/molecules26196075
Chicago/Turabian StyleXu, Mengyue, Zhili Wan, and Xiaoquan Yang. 2021. "Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems" Molecules 26, no. 19: 6075. https://doi.org/10.3390/molecules26196075
APA StyleXu, M., Wan, Z., & Yang, X. (2021). Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules, 26(19), 6075. https://doi.org/10.3390/molecules26196075