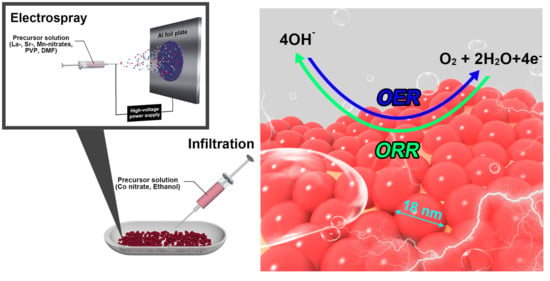
A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions: Nano-Co3O4-Deposited La0.5Sr0.5MnO3 via Infiltration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization
2.2. Rotating Ring-Disk Electrode (RRDE) Test
2.3. Four-Electron Pathway
2.4. X-Ray Photoelectron Spectroscopy (XPS) Anaylsis
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton-conducting electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-W.W.; Yoo, S.; Kim, C.; Kim, S.; Jeon, I.-Y.Y.; Shin, J.; Baek, J.-B.B.; Kim, G. Fe@N-Graphene Nanoplatelet-Embedded Carbon Nanofibers as Efficient Electrocatalysts for Oxygen Reduction Reaction. Adv. Sci. 2015, 3, 1500205. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Yu, X.; Manthiram, A. A pair of metal organic framework (MOF)-derived oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) catalysts for zinc-air batteries. Mater. Today Energy 2020, 16, 100405. [Google Scholar] [CrossRef]
- Jung, J.-I.; Park, S.; Kim, M.-G.; Cho, J. Tunable Internal and Surface Structures of the Bifunctional Oxygen Perovskite Catalysts. Adv. Energy Mater. 2015, 5, 1501560. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, O.; Kim, C.; Gwon, O.; Jeong, H.Y.; Kim, K.-H.; Shin, J.; Kim, G. Strategy for Enhancing Interfacial Effect of Bifunctional Electrocatalyst: Infiltration of Cobalt Nanooxide on Perovskite. Adv. Mater. Interfaces 2018, 5, 1800123. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, Y.; Mao, P.; Liao, K.; Zou, X.; Dai, J.; Tan, P.; Ran, R.; Zhou, W.; Ni, M.; et al. Rich atomic interfaces between sub-1 nm RuOx clusters and porous Co3O4 nanosheets boost oxygen electrocatalysis bifunctionality for advanced Zn-air batteries. Energy Storage Mater. 2020, 32, 20–29. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nagai, T.; Ioroi, T.; Arai, H.; Ogumi, Z. Bifunctional electrocatalysts of lanthanum-based perovskite oxide with Sb-doped SnO2 for oxygen reduction and evolution reactions. J. Power Sources 2020, 451, 227736. [Google Scholar] [CrossRef]
- Sadighi, Z.; Huang, J.; Qin, L.; Yao, S.; Cui, J.; Kim, J.K. Positive role of oxygen vacancy in electrochemical performance of CoMn2O4 cathodes for Li-O2 batteries. J. Power Sources 2017, 365, 134–147. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-horn, Y. A Perovskite Oxide Optimized for Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 Nanocrystals on Graphene as a Synergistic Catalyst for Oxygen Reduction Reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, H.; Yoon, K.J.; Lee, J.H.; Kim, B.K.; Choi, W.; Lee, J.H.; Hong, J. Reactions and mass transport in high temperature co-electrolysis of steam/CO2 mixtures for syngas production. J. Power Sources 2015, 280, 630–639. [Google Scholar] [CrossRef]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxides as a Bi-Functional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Behnken, J.; Yu, M.; Deng, X.; Tüysüz, H.; Harms, C.; Dyck, A.; Wittstock, G. Oxygen Reduction Reaction Activity of Mesostructured Cobalt-Based Metal Oxides Studied with the Cavity-Microelectrode Technique. ChemElectroChem 2019, 6, 3460–3467. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Shi, L.; Liu, Q.; Huang, H.; Jiao, T. Improved oxygen reduction activity on silver-modified LaMnO3-graphene via shortens the conduction path of adsorbed oxygen. RSC Adv. 2015, 5, 92096–92106. [Google Scholar] [CrossRef]
- Wang, W.; Geng, J.; Kuai, L.; Li, M.; Geng, B. Porous Mn2O3: A Low-Cost Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media with Comparable Activity to Pt/C. Chem. A Eur. J. 2016, 22, 9909–9913. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Delker, C.J.; Davis, D.J.; Kelly, M.; Brumbach, M.T.; Rodriguez, M.A.; Swartzentruber, B.S. Understanding the Effects of Cationic Dopants on α-MnO2 Oxygen Reduction Reaction Electrocatalysis. J. Phys. Chem. C 2017, 121, 2789–2797. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Agnihotri, N.; Sen, P.; De, A. Conducting CoMn2O4—PEDOT nanocomposites as catalyst in oxygen reduction reaction. Electrochim. Acta 2014, 118, 81–87. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Q.; Guo, J.; Cheng, J.; Xu, X.; Guo, H.; Li, D.; Chen, L.; Si, P.; Lou, J.; et al. Mesoporous Mn2O3 rods as a highly efficient catalyst for Li-O2 battery. J. Power Sources 2019, 435, 226833. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; et al. Graphene–Co3O4 nanocomposite as an efficient bifunctional catalyst for lithium–air batteries. J. Mater. Chem. A 2014, 2, 7188. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic bifunctional catalyst design based on perovskite oxide nanoparticles and intertwined carbon nanotubes for rechargeable zinc-air battery applications. ACS Appl. Mater. Interfaces 2015, 7, 902–910. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Wang, R.; Seo, J.M.; Choi, H.J.; Jung, S.M.; Mahmood, J.; Jeon, I.Y.; Baek, J.B. Controlled Fabrication of Hierarchically Structured Nitrogen-Doped Carbon Nanotubes as a Highly Active Bifunctional Oxygen Electrocatalyst. Adv. Funct. Mater. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Ma, C.; Ma, Z.; Sun, C.; Li, J.; Chen, L.; Kim, Y. Flowerlike Co3O4 microspheres loaded with copper nanoparticle as an efficient bifunctional catalyst for lithium-air batteries. Electrochem. Commun. 2013, 28, 13–16. [Google Scholar] [CrossRef]
- Chen, W.F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-evolution catalysts based on non-noble metal nickel-molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 2012, 51, 6131–6135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiao, H.; Wang, H.-Y.; Zhou, N.; Chen, J.; Tang, Y.-G.; Li, J.; Huang, C. Nickel Cobalt Oxide/carbon Nanotubes Hybrid as a High-performance Electrocatalyst for Metal/air Battery. Nanoscale 2014, 6, 10235–10242. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Balaya, P. Size effects and nanostructured materials for energy applications. Energy Environ. Sci. 2008, 1, 645–654. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Liu, Y.; Lin, Z.; Yan, X. Ultra-small, size-controlled Ni(OH)2 nanoparticles: Elucidating the relationship between particle size and electrochemical performance for advanced energy storage devices. NPG Asia Mater. 2015, 7, e183. [Google Scholar] [CrossRef] [Green Version]





| Abbreviations | Chemical Composition | |
|---|---|---|
| LSM | La0.5Sr0.5MnO3-δ | |
| LSM@Co3O4 | LSM@Co3O4-20 | 20 wt.% Co3O4-infiltrated LSM |
| LSM@Co3O4-25 | 25 wt.% Co3O4-infiltrated LSM | |
| Reaction Process | Thermodynamic Electrode Potential at Standard Conditions, V vs. SHE | |
|---|---|---|
| 4-electron pathway | O2 + 2H2O + 4e- → 4OH- | 0.401 |
| 2+2-electron pathway | O2 + H2O + 2e- → HO2- + OH- | −0.065 |
| HO2- + H2O + 2e- → 3OH- | 0.867 |
| Sample | Species | BE (eV) | Ratio (Co3+/Co2+) | Ratio (Mn3+/Mn4+) | Ratio (Oad/Olattice) | ||
|---|---|---|---|---|---|---|---|
| LSM | Mn | 2p3/2 | Mn3+ | 641.49 | 0.83 | ||
| Mn4+ | 643.36 | ||||||
| 2p1/2 | Mn3+ | 653.26 | |||||
| Mn4+ | 656.21 | ||||||
| O 1s | Olattice | 528.77 | 1.68 | ||||
| Oad | 528.98 | ||||||
| H2Oad | 530.96 | ||||||
| C 1s | 284.3 | ||||||
| Co3O4 | Co | 2p3/2 | Co3+ | 779.32 | 0.45 | ||
| Co2+ | 780.56 | ||||||
| 2p1/2 | Co3+ | 794.34 | |||||
| Co2+ | 795.77 | ||||||
| O 1s | Olattice | 529.57 | 2.02 | ||||
| Oad | 530.48 | ||||||
| H2Oad | 533.08 | ||||||
| C 1s | 284.3 | ||||||
| LSM@Co3O4-20 | Co | 2p3/2 | Co3+ | 779.53 | 0.68 | ||
| Co2+ | 780.84 | ||||||
| 2p1/2 | Co3+ | 794.58 | |||||
| Co2+ | 796.00 | ||||||
| Mn | 2p3/2 | Mn3+ | 641.31 | 0.77 | |||
| Mn4+ | 643.34 | ||||||
| 2p1/2 | Mn3+ | 652.93 | |||||
| Mn4+ | 655.19 | ||||||
| O 1s | Olattice | 529.33 | 2.39 | ||||
| Oad | 530.40 | ||||||
| H2Oad | 532.44 | ||||||
| C 1s | 284.3 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, G.; Manthiram, A. A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions: Nano-Co3O4-Deposited La0.5Sr0.5MnO3 via Infiltration. Molecules 2021, 26, 277. https://doi.org/10.3390/molecules26020277
Kim S, Kim G, Manthiram A. A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions: Nano-Co3O4-Deposited La0.5Sr0.5MnO3 via Infiltration. Molecules. 2021; 26(2):277. https://doi.org/10.3390/molecules26020277
Chicago/Turabian StyleKim, Seona, Guntae Kim, and Arumugam Manthiram. 2021. "A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions: Nano-Co3O4-Deposited La0.5Sr0.5MnO3 via Infiltration" Molecules 26, no. 2: 277. https://doi.org/10.3390/molecules26020277
APA StyleKim, S., Kim, G., & Manthiram, A. (2021). A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions: Nano-Co3O4-Deposited La0.5Sr0.5MnO3 via Infiltration. Molecules, 26(2), 277. https://doi.org/10.3390/molecules26020277