Construction of Charring-Functional Polyheptanazine towards Improvements in Flame Retardants of Polyurethane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PCN and Co@PCN
2.2. Fracture Surface Analysis of TPU and its Nanocomposites
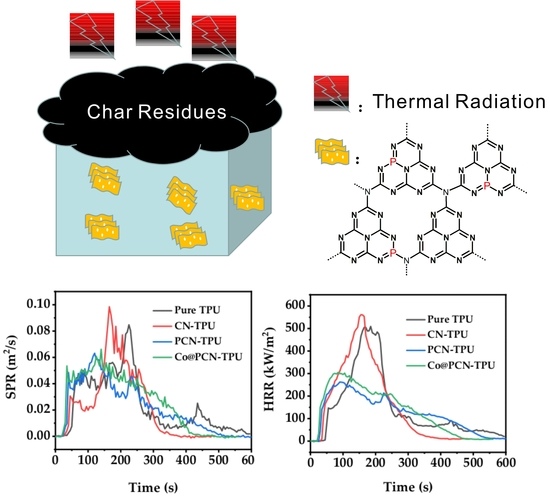
2.3. Thermal Stability and Fire Resistance of TPU and Its Composites
2.4. Evolution of Pyrolysis Gas
3. Materials and Methods
3.1. Materials
3.2. Preparation of Phosphorus-Doped Polyheptazine Nanomaterials (PCN)
3.3. Preparation of Co@PCN Nanomaterials
3.4. Preparation of Flame-Retarding TPU
3.5. Characterization Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Y.; Miao, J.; Yang, X.; Zhu, Y.; Wang, G. Fabrication of polyphosphazene covalent triazine polymer with excellent flame retardancy and smoke suppression for epoxy resin. Chem. Eng. J. 2020, 385, 123830. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, W.; Yang, M.; Chen, G.; Hong, W.; Yu, D.; Zheng, Z.; Chen, X. Preparation and flame-retardant mechanism of polyheptazine/PA6 nanocmposites. Polym. 2019, 182, 121810. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron-graphene and melamine salt of pentaerythritol phosphate on flame retardant thermoplastic polyurethane. Polym. Adv. Technol. 2016, 27, 1508–1516. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Wang, W.; Wang, Y.; Jiao, C. Fire-safe agent integrated with oyster shell and melamine polyphosphate for thermoplastic polyurethane. Polym. Adv. Technol. 2019, 30, 1576–1588. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, W.; Liu, Y.; Xia, J.; Wu, Q.; Xu, W. Preparation and Characterization of Flame-Retardant Melamine Cyanu-rate/Polyamide 6 Nanocomposites by in Situ Polymerization. J. Appl. Polym. Sci. 2009, 113, 2109–2116. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, S.; Zhou, K.; Bao, C.; Yu, B.; Qian, X.; Wang, B.; Hong, N.; Wen, P.; Gui, Z.; et al. Influence of g-C3N4 Nanosheets on Thermal Stability and Mechanical Properties of Biopolymer Electrolyte Nanocomposite Films: A Novel Investigation. ACS Appl. Mater. Interfaces 2014, 6, 429–437. [Google Scholar] [CrossRef]
- Shi, Y.; Long, Z.; Zhou, K.; Gui, Z.; Yu, B.; Yuen, K.K.R.; Hu, Y. Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J. Mater. Chem. A 2015, 3, 17064–17073. [Google Scholar] [CrossRef]
- Lu, S.; Hong, W.; Chen, X. Nanoreinforcements of Two-Dimensional Nanomaterials for Flame Retardant Polymeric Composites: An Overview. Adv. Polym. Technol. 2019, 2019, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yunsheng, Y.; Wen, Y.; Ye, Y.S.; Zhou, X.; Xie, X.; Mai, Y.-W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; You, J.; Li, F.; Fan, Z.; Wang, F.; Liu, D.; Gui, J. A simple and efficient method to prepare a phosphorus modified g-C3N4 visible light photocatalyst. RSC Adv. 2014, 4, 21657–21663. [Google Scholar] [CrossRef]
- Velencoso, M.-M.; Battig, A.; Markwart, J.-C.; Schartel, B.; Wurm, F.-R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Flame Retardancy Task. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.; Jiang, H.; Chen, X. Properties of fire agent integrated with molecular sieve and tetrafluoroborate ionic liquid in thermoplastic polyurethane elastomer. Polym. Adv. Technol. 2019, 30, 2159–2167. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants. Molecules 2020, 25, 4963. [Google Scholar] [CrossRef]
- Huang, S.-C.; Deng, C.; Chen, H.; Li, Y.-M.; Zhao, Z.-Y.; Wang, S.-X.; Wang, Y.-Z. Novel Ultrathin Layered Double Hydroxide Nanosheets with In Situ Formed Oxidized Phosphorus as Anions for Simultaneous Fire Resistance and Mechanical Enhancement of Thermoplastic Polyurethane. ACS Appl. Polym. Mater. 2019, 1, 1979–1990. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, K.; Zhou, K.; Zhao, S.; Shi, C. Synergistic effect between phosphorus tailings and aluminum hypophosphite in flame-retardant thermoplastic polyurethane composites. Polym. Adv. Technol. 2019, 30, 2480–2487. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, C.; Zhou, K.; Xuan, X.; Shi, C. Synergistic effect between solid wastes and intumescent flame retardant on flammability and smoke suppression of thermoplastic polyurethane composites. Polym. Adv. Technol. 2020, 31, 4–14. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Liquid-Crystal and Fire-Retardant Properties of New Hexasubstituted Cyclotri-phosphazene Compounds with Two Schiff Base Linking Units. Molecules 2020, 25, 2122. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Fang, H.-B.; Zhang, X.-H.; Wu, J.; Li, N.; Zheng, Y.-Z.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-Doped Tubular G-C3N4 with Surface Carbon Defects: Uni-versal Synthesis and Enhanced Visible-Light Photocatalytic Hydrogen Production. Appl. Catal. B 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, S.; Ge, R.; Feng, L.; Yu, Y.; Li, Y.; Li, W. Cobalt Oxide Supported on Phosphorus-Doped g-C3N4 as an Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 4718–4729. [Google Scholar] [CrossRef]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon Quantum Dots Supported AgI/ZnO/Phosphorus Doped Graphitic Carbon Nitride as Z-Scheme Photocatalyst for Efficient Photodegradation of 2, 4-Dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Zhu, Z.; Yan, M.; Zhou, Y.; Wang, J.; Liu, Y.; Wang, J. Insight Into Highly Efficient Simultaneous Photocatalytic Removal of Cr(Vi) and 2,4-Diclorophenol Under Visible Light Irradiation by Phosphorus Doped Porous Ul-trathin G-C3N4 Nanosheets From Aqueous Media: Performance and Reaction Mechanism. Appl. Catal. B 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Rong, M.; Cai, Z.; Xie, L.; Lin, C.; Song, X.; Luo, F.; Wang, Y.; Chen, X. Study on the Ultrahigh Quantum Yield of Fluorescent P,O-g-C3N4Nanodots and its Application in Cell Imaging. Chem. A Eur. J. 2016, 22, 9387–9395. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, B.; Li, Q.; Li, S.; Zhang, S. Synergistic effect of metal oxides on the flame retardancy and thermal degradation of novel intumescent flame-retardant thermoplastic polyurethanes. J. Appl. Polym. Sci. 2011, 121, 1951–1960. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Penverne, C.; Bourbigot, S. Thermal Stability and Fire Properties of Salen and Metallosalens as Fire Retardants in Thermoplastic Polyurethane (TPU). Materials 2017, 10, 665. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Hu, Y.; Zhou, K.; Yuen, R.K.; Gui, Z.; Saihua, J.; Jiang, S. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef]
- Shi, Y.; Gui, Z.; She, S.; Zhu, Y.; Yu, B.; Yuen, K.K.R.; Liu, H.; Hu, Y. Enhanced thermal stability of polystyrene by graphitic carbon nitride/spinel ZnCo 2 O 4 nanohybrids and the catalytic mechanism investigation. RSC Adv. 2015, 5, 41835–41838. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. Preparation of Metal–Organic Frameworks and Their Application as Flame Retardants for Polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. [Google Scholar] [CrossRef]
- Xu, W.; Chen, R.; Du, Y.; Wang, G. Design Water-Soluble Phenolic/Zeolitic Imidazolate Framework-67 Flame Retardant Coating Via Layer-by-Layer Assembly Technology: Enhanced Flame Retardancy and Smoke Suppression of Flexible Polyure-thane Foam. Polym. Degrad. Stabil. 2020, 176, 109152. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y.; Jiang, S.; Tang, G. The influence of cobalt oxide–graphene hybrids on thermal degradation, fire hazards and mechanical properties of thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2016, 88, 10–18. [Google Scholar] [CrossRef]
- Tan, H.; Tang, J.; Henzie, J.; Li, Y.; Xu, X.; Chen, T.; Wang, Z.; Wang, J.; Ide, Y.; Bando, Y.; et al. Assembly of Hollow Carbon Nanospheres on Graphene Nanosheets and Creation of Iron–Nitrogen-Doped Porous Carbon for Oxygen Reduction. ACS Nano 2018, 12, 5674–5683. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Mahmood, N.; Pan, L.; Huang, Z.-F.; Lv, Z.; Zhang, J.; Butt, F.K.; Shen, G.; Zhang, X.; Dou, S.X.; et al. Efficient water oxidation through strongly coupled graphitic C3N4 coated cobalt hydroxide nanowires. J. Mater. Chem. A 2016, 4, 12940–12946. [Google Scholar] [CrossRef]
- Xue, F.; Si, Y.; Wang, M.; Liu, M.; Guo, L. Toward efficient photocatalytic pure water splitting for simultaneous H2 and H2O2 production. Nano Energy 2019, 62, 823–831. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-Doped Carbon Nitride Solid: Enhanced Electrical Conductivity and Photocurrent Generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand New P-Doped G-C3N4: En-hanced Photocatalytic Activity for H2 Evolution and Rhodamine B Degradation under Visible Light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Zhao, D.; Song, M.; Guo, P.; Shen, S.; Li, D.; Yang, S. Enhanced photocatalytic hydrogen evolution by partially replaced corner-site C atom with P in g-C3N4. Appl. Catal. B Environ. 2019, 244, 486–493. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, D.; Zhou, H.; Pi, M.; Wang, X.; Chen, S. Coupling P Nanostructures with P-Doped g-C3N4 As Efficient Visible Light Photocatalysts for H2 Evolution and RhB Degradation. ACS Sustain. Chem. Eng. 2018, 6, 6342–6349. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of aromatic alcohols in water by using P-doped g-C3N4. Appl. Catal. B Environ. 2018, 220, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Xu, C.; Zhou, N.; Chen, H.; Jiang, F. Metal-free black phosphorus nanosheets-decorated graphitic carbon nitride nanosheets with C P bonds for excellent photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2018, 221, 27–35. [Google Scholar] [CrossRef]
- Wang, S.; He, F.; Zhao, X.; Zhang, J.; Ao, Z.; Wu, H.; Yin, Y.; Shi, L.; Xu, X.; Zhao, C.; et al. Phosphorous doped carbon nitride nanobelts for photodegradation of emerging contaminants and hydrogen evolution. Appl. Catal. B Environ. 2019, 257, 117931. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Yan, Q.; Dong, P.; Wang, Y.; Liu, F.; Li, L. Nitrogen Defects-Rich 0D/2D α-Fe2O3/g-C3N4 Z-Scheme Pho-tocatalyst for Enhanced Photooxidation and H2 Evolution Efficiencies. Nano 2018, 13, 1850086. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Qu, L. Graphitic Carbon Nitride/Nitrogen-Rich Carbon Nanofibers: Highly Efficient Photocatalytic Hydrogen Evolution without Cocatalysts. Angew. Chem. Int. Ed. 2016, 55, 10849–10853. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Choi, J.-Y.; Zamani, P.; Jiang, G.; Hoque, A.; Hassan, F.M.; Chen, Z. Co–N Decorated Hierarchically Porous Graphene Aerogel for Efficient Oxygen Reduction Reaction in Acid. ACS Appl. Mater. Interfaces 2016, 8, 6488–6495. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous Phosphorus-Doped g-C3N4 Nanostructured Flowers with Superior Photocatalytic Hydrogen Evolution Performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Fang, L.; Xu, H.; Zhang, H.; Gu, X.; Wang, Y. Probing the Crystal Plane Effect of Co3O4 for Enhanced Elec-trocatalytic Performance Toward Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 27736–27744. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Huang, M.; Wang, S.; Li, X.; Zhu, H. Cobalt and nickel selenide nanowalls anchored on graphene as bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 14789–14795. [Google Scholar] [CrossRef]
- Tao, L.; Lin, C.-Y.; Dou, S.; Feng, S.; Chen, D.; Liu, D.; Huo, J.; Xia, Z.; Wang, S. Creating coordinatively unsaturated metal sites in metal-organic-frameworks as efficient electrocatalysts for the oxygen evolution reaction: Insights into the active centers. Nano Energy 2017, 41, 417–425. [Google Scholar] [CrossRef]
- Pylypenko, S.; Mukherjee, S.; Olson, T.S.; Atanassov, P. Non-platinum oxygen reduction electrocatalysts based on pyrolyzed transition metal macrocycles. Electrochim. Acta 2008, 53, 7875–7883. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef]
- Tao, K.; Li, J.; Xu, L.; Zhao, X.; Xue, L.; Fan, X.; Yan, Q. A novel phosphazene cyclomatrix network polymer: Design, synthesis and application in flame retardant polylactide. Polym. Degrad. Stab. 2011, 96, 1248–1254. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Novel cyclotriphosphazene-based epoxy compound and its application in halogen-free epoxy thermosetting systems: Synthesis, curing behaviors, and flame retardancy. Polym. Degrad. Stab. 2014, 103, 96–112. [Google Scholar] [CrossRef]
- Formicola, C.; De Fenzo, A.; Zarrelli, M.; Giordano, M.; Antonucci, V. Zinc-based compounds as smoke suppressant agents for an aerospace epoxy matrix. Polym. Int. 2010, 60, 304–311. [Google Scholar] [CrossRef]
- Fei, B.; Tawiah, B.; Wang, L.-Q.; Yuen, A.; Zhang, Z.-C.; Shen, L.-L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Zhang, Y.; Cai, W.; Yao, C.; Hu, Y.; Hu, W. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef]
- Wang, S.; Gao, R.; Zhou, K. The influence of cerium dioxide functionalized reduced graphene oxide on reducing fire hazards of thermoplastic polyurethane nanocomposites. J. Colloid Interface Sci. 2019, 536, 127–134. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Fu, L.; Liu, C.; Yu, B.; Yang, F.; Hu, Y. Sodium Alginate-Templated Synthesis of g-C3N4/Carbon Spheres/Cu Ternary Nanohybrids for Fire Safety Application. J. Colloid Interface Sci. 2019, 539, 1–10. [Google Scholar] [CrossRef]
- Wei, W.-C.; Deng, C.; Huang, S.-C.; Wei, Y.-X.; Wang, Y.-Z. Nickel-Schiff base decorated graphene for simultaneously enhancing the electroconductivity, fire resistance, and mechanical properties of a polyurethane elastomer. J. Mater. Chem. A 2018, 6, 8643–8654. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Cai, W.; Yuan, B.; Zhang, Y.; Guo, W.; Hu, W.; Song, L. Atherton–Todd reaction assisted synthesis of functionalized multicomponent MoSe2/CNTs nanoarchitecture towards the fire safety enhancement of polymer. Compos. Part A Appl. Sci. Manuf. 2018, 112, 271–282. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Pan, Y.; Guo, W.; Mu, X.; Feng, X.; Yuan, B.; Wang, X.; Hu, Y. Mussel-Inspired Functionalization of Elec-trochemically Exfoliated Graphene: Based on Self-Polymerization of Dopamine and its Suppression Effect on the Fire Hazards and Smoke Toxicity of Thermoplastic Polyurethane. J. Hazard. Mater. 2018, 352, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, C.; Mu, X.; Cai, W.; Liu, L.; Zhou, X.; Hu, W.; Hu, Y. Construction of multifunctional MoSe2 hybrid towards the simultaneous improvements in fire safety and mechanical property of polymer. J. Hazard. Mater. 2018, 352, 36–46. [Google Scholar] [CrossRef] [PubMed]











| Samples | C, at.% | N, at.% | O, at.% | P, at.% | Co, at.% |
|---|---|---|---|---|---|
| CN | 41.74 | 56.93 | 1.33 | - | - |
| PCN | 25.90 | 33.30 | 32.54 | 8.14 | - |
| Co@PCN | 32.20 | 43.52 | 11.45 | 10.05 | 2.78 |
| Sample | T−5 1 | T−50 2 | T−max 3 | Residues at 750 °C (wt%) |
|---|---|---|---|---|
| CN | 558 | 663 | 686 | 0 |
| PCN | 247 | 683 | 699 | 26.3 |
| Co@PCN | 541 | 738 | 685 | 47.1 |
| Sample | T−5 | T−50 | T−max | Residues at 700 °C (wt%) |
|---|---|---|---|---|
| Pure TPU | 306 | 392 | 407 | 4.4 |
| CN-TPU | 300 | 391 | 399 | 5.2 |
| PCN-TPU | 287 | 393 | 404 | 9.5 |
| Co@PCN-TPU | 310 | 402 | 410 | 10.7 |
| Sample | pHRR 1 (W/g) | pHRR 2 (W/g) | THR (MJ/m2) | pSPR (m2/s) | Residues (%) |
|---|---|---|---|---|---|
| Pure TPU | 407.8 | 509.4 | 86.34 | 0.0847 | 10.18 |
| CN-TPU | 373.2 | 561.4 | 82.31 | 0.0985 | 7.25 |
| PCN-TPU | 269.9 | 262.2 | 74.01 | 0.0631 | 17.04 |
| Co@PCN-TPU | 341.0 | 305.4 | 76.68 | 0.0662 | 14.08 |
| Sample | CO2 (kg/kg) | CO (kg/kg) | CO2/CO | UL-94 |
|---|---|---|---|---|
| Pure TPU | 61.0 | 1.36 | 44.9 | V-2 |
| CN-TPU | 56.9 | 1.53 | 37.2 | V-2 |
| PCN-TPU | 48.4 | 1.63 | 29.7 | V-2 |
| Co@PCN-TPU | 51.4 | 1.85 | 27.8 | V-2 |
| Recipes | Residues (wt%) 1 | pHRR | pSRR | Year and Ref. |
|---|---|---|---|---|
| 2 wt% CTAB-Ti3C2 | 1.3→6.1 | −51.2% | −57.1% | 2019 [56] |
| 3 wt% CPBN | 4.1→10.2 | −35.6% | - | 2019 [57] |
| 2 wt% CeO2/rGO | 7.3→9.7 | −41.1% | −50.0% | 2019 [58] |
| 2 wt% CSACS-C | 0.3→2.0 | −35.9% | - | 2019 [59] |
| 2 wt% Co3O4-GNS | 2.8→8.5 | −16.4% | - | 2016 [32] |
| 1.5 wt% rGO-Salen-Ni | 5.5→9.0 | −43.1% | - | 2018 [60] |
| 3 wt% HC-Mo | 3.4→6.6 | −38.3% | −32.1% | 2018 [61] |
| 3 wt% PCN (This work) | 4.4→9.5 | −48.5% | −25.5% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Shen, B.; Chen, X. Construction of Charring-Functional Polyheptanazine towards Improvements in Flame Retardants of Polyurethane. Molecules 2021, 26, 340. https://doi.org/10.3390/molecules26020340
Lu S, Shen B, Chen X. Construction of Charring-Functional Polyheptanazine towards Improvements in Flame Retardants of Polyurethane. Molecules. 2021; 26(2):340. https://doi.org/10.3390/molecules26020340
Chicago/Turabian StyleLu, Shaolin, Botao Shen, and Xudong Chen. 2021. "Construction of Charring-Functional Polyheptanazine towards Improvements in Flame Retardants of Polyurethane" Molecules 26, no. 2: 340. https://doi.org/10.3390/molecules26020340
APA StyleLu, S., Shen, B., & Chen, X. (2021). Construction of Charring-Functional Polyheptanazine towards Improvements in Flame Retardants of Polyurethane. Molecules, 26(2), 340. https://doi.org/10.3390/molecules26020340