Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytokinins’ Oxygen Radical Absorbance Capacity (ORAC)
2.2. Differentiation of SH-SY5Y Cells
2.3. Cytotoxicity of Cytokinins towards Neuron-like SH-SY5Y Cells
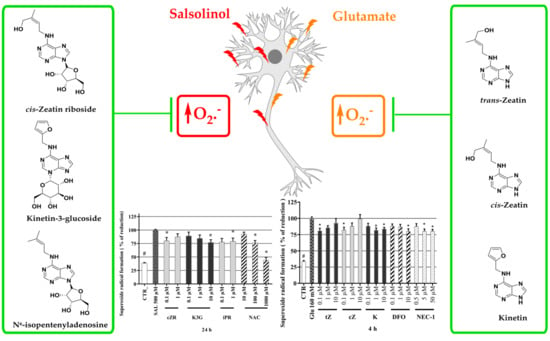
2.4. Identification of Neuroprotective Cytokinins in the SAL-induced Model of PD
2.5. Cytokinins Decrease SAL-induced Superoxide Radical Formation
2.6. Anti-Apoptotic Effects of Cytokinins Determined by Caspase-3/7 Activity Measurements
2.7. Identification of Cytokinin Neuroprotective Activity in the Glutamate-induced Cell Death Model
2.8. Effects of Cytokinins on Glu-induced Oxidative Stress in SH-SY5Y cells
2.9. Effects of Cytokinins on Caspase-3/7 Activity in the Glu-induced Cell Death Model
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. ORAC Radical Scavenging Activity Assays
4.3. SH-SY5Y Cell Culture
4.4. Microscopy
4.5. Cell Membrane Staining (Neurite Outgrowth kit, Invitrogen™)
4.6. Cell Treatment
4.7. Cell Viability and Cell Death
4.8. Measurement of Oxidative Stress by the Dihydroethidium (DHE) assay
4.9. Measurement of Caspase-3/7 Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ Can. Med Assoc. J. 2016, 188, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovic, J. Progression of Parkinson disease: Are we making progress in charting the course? Arch. Neurol. 2005, 62, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Alam, Z.I.; Daniel, S.E.; Lees, A.J.; Marsden, D.C.; Jenner, P.; Halliwell, B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997, 69, 1326–1329. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J. Neural Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef]
- Dantuma, N.P.; Bott, L.C. The ubiquitin-proteasome system in neurodegenerative diseases: Precipitating factor, yet part of the solution. Front. Mol. Neurosci. 2014, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Cookson, M.R.; Bandmann, O. Parkinson’s disease: Insights from pathways. Hum. Mol. Genet. 2010, 19, R21–R27. [Google Scholar] [CrossRef] [Green Version]
- Rinne, U.K. Problems associated with long-term levodopa treatment of Parkinson’s disease. Acta Neurol. Scand. Suppl. 1983, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieber, J.J. Tribute to Folke Skoog: Recent Advances in our Understanding of Cytokinin Biology. J. Plant Growth Regul. 2002, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Voller, J.; Maková, B.; Kadlecová, A.; Gonzalez, G.; Strnad, M. Plant Hormone Cytokinins for Modulating Human Aging and Age-Related Diseases. In Hormones in Ageing and Longevity; Rattan, S., Sharma, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 311–335. [Google Scholar]
- Rattan, S.I.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res 2005, 8, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Z.; Yang, X. Kinetin protects against lipid peroxidation and improves antioxidant status in cultured astrocytes and mouse brain exposed to D-galactose. Afr. J. Biotechnol. 2011, 10, 11721–11727. [Google Scholar]
- Hertz, N.T.; Berthet, A.; Sos, M.L.; Thorn, K.S.; Burlingame, A.L.; Nakamura, K.; Shokat, K.M. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell 2013, 154, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Liu, D.; Zheng, Y.; Hao, C.; Li, H.; Ouyang, W. Neuroprotective Effects of Kinetin Against Glutamate-Induced Oxidative Cytotoxicity in HT22 Cells: Involvement of Nrf2 and Heme Oxygenase-1. Neurotox. Res. 2018, 33, 725–737. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Yang, Y.-C.; Huang, C.-L.; Kuo, T.-Y.; Lin, J.-H.; Yang, D.-M.; Huang, N.-K. When Cytokinin, a Plant Hormone, Meets the Adenosine A2A Receptor: A Novel Neuroprotectant and Lead for Treating Neurodegenerative Disorders? PLoS ONE 2012, 7, e38865. [Google Scholar] [CrossRef] [Green Version]
- Brizzolari, A.; Marinello, C.; Carini, M.; Santaniello, E.; Biondi, P.A. Evaluation of the antioxidant activity and capacity of some natural N6-substituted adenine derivatives (cytokinins) by fluorimetric and spectrophotometric assays. J. Chromatogr. B 2016, 1019, 164–168. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Neudecker, B.A.; DiNardo, J.C.; Lewis Ii, J.A.; Maibach, H.I. Idebenone: A new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J. Cosmet. Dermatol. 2005, 4, 10–17. [Google Scholar] [CrossRef]
- Dassano, A.; Mancuso, M.; Giardullo, P.; De Cecco, L.; Ciuffreda, P.; Santaniello, E.; Saran, A.; Dragani, T.A.; Colombo, F. N(6)-isopentenyladenosine and analogs activate the NRF2-mediated antioxidant response. Redox Biol. 2014, 2, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Kurnik-Łucka, M.; Panula, P.; Bugajski, A.; Gil, K. Salsolinol: An Unintelligible and Double-Faced Molecule—Lessons Learned from In Vivo and In Vitro Experiments. Neurotox. Res. 2018, 33, 485–514. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chai, S.; Ju, Y.; Hou, L.; Zhao, H.; Ma, W.; Li, T.; Sheng, J.; Shi, W. Pu-erh Tea Protects the Nervous System by Inhibiting the Expression of Metabotropic Glutamate Receptor 5. Mol. Neurobiol. 2017, 54, 5286–5299. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [Green Version]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Rárová, L.; Steigerová, J.; Kvasnica, M.; Bartůněk, P.; Křížová, K.; Chodounská, H.; Kolář, Z.; Sedlák, D.; Oklestkova, J.; Strnad, M. Structure activity relationship studies on cytotoxicity and the effects on steroid receptors of AB-functionalized cholestanes. J. Steroid Biochem. Mol. Biol. 2016, 159, 154–169. [Google Scholar] [CrossRef]
- Voller, J.; Zatloukal, M.; Lenobel, R.; Dolezal, K.; Béres, T.; Krystof, V.; Spíchal, L.; Niemann, P.; Dzubák, P.; Hajdúch, M.; et al. Anticancer activity of natural cytokinins: A structure-activity relationship study. Phytochemistry 2010, 71, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Texel, S.J.; Zhang, J.; Camandola, S.; Unger, E.L.; Taub, D.D.; Koehler, R.C.; Harris, Z.L.; Mattson, M.P. Ceruloplasmin Deficiency Reduces Levels of Iron and BDNF in the Cortex and Striatum of Young Mice and Increases Their Vulnerability to Stroke. PLoS ONE 2011, 6, e25077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Yang, H.; Yang, M.; Yanagisawa, D.; Bellier, J.-P.; Mori, M.; Takahata, S.; Nonaka, T.; Zhao, S.; Tooyama, I. Mitochondrial ferritin protects SH-SY5Y cells against H2O2-induced oxidative stress and modulates α-synuclein expression. Exp. Neurol. 2017, 291, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.; Aki, T.; Funakoshi, T.; Noritake, K.; Unuma, K.; Uemura, K. Necrosis in human neuronal cells exposed to paraquat. J. Toxicol. Sci. 2018, 43, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Eguchi, Y.; Imagawa, Y.; Akai, S.; Mochizuki, H.; Tsujimoto, Y. MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov. 2017, 3, 17013. [Google Scholar] [CrossRef] [Green Version]
- Wanpen, S.; Govitrapong, P.; Shavali, S.; Sangchot, P.; Ebadi, M. Salsolinol, a dopamine-derived tetrahydroisoquinoline, induces cell death by causing oxidative stress in dopaminergic SH-SY5Y cells, and the said effect is attenuated by metallothionein. Brain Res. 2004, 1005, 67–76. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
- Othman, E.M.; Naseem, M.; Awad, E.; Dandekar, T.; Stopper, H. The Plant Hormone Cytokinin Confers Protection against Oxidative Stress in Mammalian Cells. PLoS ONE 2016, 11, e0168386. [Google Scholar] [CrossRef]
- Yap, Y.; Omasanggar, R.; Koh, Y.L.; Yew, M.Y.; Lai, H.T.; Ling, A.P.K.; Chye, S.M.; Ng, K.Y.; Koh, R.Y. Neurotoxic effect of salsolinol through oxidative stress induction and Nrf2-Keap1 signalling regulation. J. Chem. Pharm. Res. 2016, 8, 30–38. [Google Scholar]
- Bindokas, V.P.; Jordan, J.; Lee, C.C.; Miller, R.J. Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J. Neurosci. 1996, 16, 1324–1336. [Google Scholar] [CrossRef]
- Carter, W.O.; Narayanan, P.K.; Robinson, J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 1994, 55, 253–258. [Google Scholar] [PubMed]
- Wanpen, S.; Kooncumchoo, P.; Shavali, S.; Govitrapong, P.; Ebadi, M. Salsolinol, an endogenous neurotoxin, activates JNK and NF-kappaB signaling pathways in human neuroblastoma cells. Neurochem. Res. 2007, 32, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Czerpak, R. N6-benzyladenine and kinetin influence antioxidative stress parameters in human skin fibroblasts. Mol. Cell. Biochem. 2016, 413, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, S.; Czapski, G. SOD-like activity studies of cytokinin-copper(II) complexes. Free Radic. Res. Commun. 1991, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Trávníček, Z.; Herchel, R.; Popa, I.; Suchý, P.; Vančo, J. Dinuclear copper(II) complexes containing 6-(benzylamino)purines as bridging ligands: Synthesis, characterization, and in vitro and in vivo antioxidant activities. J. Inorg. Biochem. 2009, 103, 432–440. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, F.; Bubacco, L.; Whitworth, A.J.; Bisaglia, M. Superoxide Radical Dismutation as New Therapeutic Strategy in Parkinson’s Disease. Aging Dis. 2018, 9, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Lalkovičová, M.; Danielisová, V. Neuroprotection and antioxidants. Neural Regen. Res. 2016, 11, 865–874. [Google Scholar] [CrossRef]
- Surendran, S.; Raja Sankar, S. Parkinson’s disease: Oxidative stress and therapeutic approaches. Neurol. Sci. 2010, 31, 531–540. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Bollimuntha, S.; Ebadi, M.; Singh, B.B. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006, 1099, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jantas, D.; Piotrowski, M.; Lason, W. An Involvement of PI3-K/Akt Activation and Inhibition of AIF Translocation in Neuroprotective Effects of Undecylenic Acid (UDA) Against Pro-Apoptotic Factors-Induced Cell Death in Human Neuroblastoma SH-SY5Y Cells. J. Cell. Biochem. 2015, 116, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Tamas, A.; Reglödi, D.; Tizabi, Y. PACAP Protects Against Salsolinol-Induced Toxicity in Dopaminergic SH-SY5Y Cells: Implication for Parkinson’s Disease. J. Mol. Neurosci. 2013, 50, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Anand, P.; Kuang, A.; Akhtar, F.; Scofield, V.L. N-Acetylcysteine in Combination with IGF-1 Enhances Neuroprotection against Proteasome Dysfunction-Induced Neurotoxicity in SH-SY5Y Cells. Parkinsons Dis. 2016, 2016, 6564212. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Meng, Z.; Zhang, G.; Xing, Y.; Feng, L.; Fan, S.; Fan, F.; Buren, B.; Liu, Q. N-acetylcysteine relieves oxidative stress and protects hippocampus of rat from radiation-induced apoptosis by inhibiting caspase-3. Biomed. Pharmacother. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Rakshit, J.; Mallick, A.; Roy, S.; Sarbajna, A.; Dutta, M.; Bandyopadhyay, J. Iron-Induced Apoptotic Cell Death and Autophagy Dysfunction in Human Neuroblastoma Cell Line SH-SY5Y. Biol. Trace Elem. Res. 2019, 193, 138–151. [Google Scholar] [CrossRef]
- Rakshit, J.; Priyam, A.; Gowrishetty, K.K.; Mishra, S.; Bandyopadhyay, J. Iron chelator Deferoxamine protects human neuroblastoma cell line SH-SY5Y from 6-Hydroxydopamine-induced apoptosis and autophagy dysfunction. J. Trace Elem. Med. Biol. 2020, 57, 126406. [Google Scholar] [CrossRef]
- Ma, X.W.; Guo, R.Y. Dose-dependent effect of Curcuma longa for the treatment of Parkinson’s disease. Exp. Ther. Med. 2017, 13, 1799–1805. [Google Scholar] [CrossRef] [Green Version]
- Naoi, M.; Maruyama, W.; Takahashi, T.; Akao, Y.; Nakagawa, Y. Involvement of endogenous N-methyl(R)salsolinol in Parkinson’s disease: Induction of apoptosis and protection by (-)deprenyl. In Advances in Research on Neurodegeneration; Mizuno, Y., Calne, D.B., Horowski, R., Poewe, W., Riederer, P., Youdim, M.B.H., Eds.; Springer: Vienna, Austria, 2000; pp. 111–121. [Google Scholar]
- Kulikov, A.V.; Rzhaninova, A.A.; Goldshtein, D.V.; Boldyrev, A.A. Expression of NMDA receptors in multipotent stromal cells of human adipose tissue under conditions of retinoic acid-induced differentiation. Bull. Exp. Biol. Med. 2007, 144, 626–629. [Google Scholar] [CrossRef]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Shi, X.; Lu, L.; Jiang, Y.; Liu, B. Stimulus-dependent neuronal cell responses in SH-SY5Y neuroblastoma cells. Mol. Med. Rep. 2016, 13, 2215–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, M.P.; Lieberknecht, V.; Ramos-Hryb, A.B.; Olescowicz, G.; Ludka, F.K.; Tasca, C.I.; Gabilan, N.H.; Rodrigues, A.L.S. Creatine affords protection against glutamate-induced nitrosative and oxidative stress. Neurochem. Int. 2016, 95, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zille, M.; Kumar, A.; Kundu, N.; Bourassa, M.W.; Wong, V.S.C.; Willis, D.; Karuppagounder, S.S.; Ratan, R.R. Ferroptosis in Neurons and Cancer Cells Is Similar But Differentially Regulated by Histone Deacetylase Inhibitors. eNeuro 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Jantas, D.; Chwastek, J.; Grygier, B.; Lasoń, W. Neuroprotective Effects of Necrostatin-1 Against Oxidative Stress–Induced Cell Damage: An Involvement of Cathepsin D Inhibition. Neurotox. Res. 2020, 37, 525–542. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.W.; Zhang, L.; Zhu, S.J.; Chen, W.C.; Mei, B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci. Bull. 2010, 26, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Liu, C.-X.; Song, R.; Li, Q.-L. Ferrostatin-1 protects HT-22 cells from oxidative toxicity. Neural Regen. Res. 2020, 15, 528–536. [Google Scholar]
- Xu, X.; Chua, C.C.; Kong, J.; Kostrzewa, R.M.; Kumaraguru, U.; Hamdy, R.C.; Chua, B.H. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J. Neurochem. 2007, 103, 2004–2014. [Google Scholar] [CrossRef]
- Shirlee, T.; David, S.; Pamela, M. Oxytosis: A Novel Form of Programmed Cell Death. Curr. Top. Med. Chem. 2001, 1, 497–506. [Google Scholar] [CrossRef]
- Nikolova, S.; Lee, Y.S.; Lee, Y.-S.; Kim, J.-A. Rac1-NADPH oxidase-regulated generation of reactive oxygen species mediates glutamate-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic. Res. 2005, 39, 1295–1304. [Google Scholar] [CrossRef]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.; Culmsee, C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef]
- Olsen, A.; Siboska, G.E.; Clark, B.F.C.; Rattan, S.I.S. N6-Furfuryladenine, Kinetin, Protects against Fenton Reaction-Mediated Oxidative Damage to DNA. Biochem. Biophys. Res. Commun. 1999, 265, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Kaur, J.; Rattan, S.I.S. Increased longevity of kinetin-fed Zaprionus fruitflies is accompanied by their reduced fecundity and enhanced catalase activity. IUBMB Life 1997, 41, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Jeong, C.H.; Choi, S.G.; Chun, J.Y.; Kim, Y.J.; Lee, J.; Shin, D.H.; Heo, H.J. Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J. Med. Food 2009, 12, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Han, A.R.; Kim, E.-A.; Yang, J.W.; Ahn, J.-Y.; Na, J.-M.; Cho, S.-W. KHG21834 attenuates glutamate-induced mitochondrial damage, apoptosis, and NLRP3 inflammasome activation in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2019, 856, 172412. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, T.N.; Yayla, M.; Halici, Z.; Cadirci, E.; Polat, B.; Kose, D. Protective effect of 5-HT7 receptor activation against glutamate-induced neurotoxicity in human neuroblastoma SH-SY5Y cells via antioxidative and antiapoptotic pathways. Neurotoxicol. Teratol. 2019, 72, 22–28. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Liu, P.; Chen, X.; Guo, D.-H.; Li, Q.-S.; Rahman, K. Protection of SH-SY5Y Neuronal Cells from Glutamate-Induced Apoptosis by 3,6′-Disinapoyl Sucrose, a Bioactive Compound Isolated from Radix Polygala. J. Biomed. Biotechnol. 2012, 2012, 728342. [Google Scholar] [CrossRef] [Green Version]
- Geng, N.; Shi, B.J.; Li, S.L.; Zhong, Z.Y.; Li, Y.C.; Xua, W.L.; Zhou, H.; Cai, J.H. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3826–3836. [Google Scholar]
- Krishnamurthy, P.; Mays, J.; Bijur, G.; Johnson, G. Transient oxidative stress in SH-SY5Y human neuroblastoma cells results in caspase dependent and independent cell death and tau proteolysis. J. Neurosci. Res. 2000, 61, 515–523. [Google Scholar] [CrossRef]
- Stone, W.L.; Qui, M.; Smith, M. Lipopolysaccharide enhances the cytotoxicity of 2-chloroethyl ethyl sulfide. BMC Cell Biol. 2003, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.A.; Stamm, N.B.; Patel, B.K.R. One-step cellular caspase-3/7 assay. BioTechniques 2003, 34, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]








| R1 | R2 | R3 | R4 | Trivial Name | Abbreviation |
|---|---|---|---|---|---|
 | - | - | H | N6-isopentenyladenine | iP |
| - | - | ribosyl | N6-isopentenyladenosine | iPR | |
 | - | - | H | trans-zeatin | tZ |
| - | - | ribosyl | t-zeatin riboside | tZR | |
| - | glycosyl | - | t-zeatin-7-glucoside | tZ7G | |
| - | - | glycosyl | t-zeatin-9-glucoside | tZ9G | |
| - | - | ribonucleotide | tZR-5‘-monophosphate | tZMP | |
 | - | - | - | t-zeatin-O-glucoside | tZOG |
 | - | - | H | cis-zeatin | cZ |
| - | - | ribosyl | c-zeatin riboside | cZR | |
 | - | - | glycosyl | c-zeatin-9-glucoside | cZ9G |
| - | - | ribonucleotide | cZR-5‘-monophosphate | cZMP | |
 | - | - | - | c-zeatin-O-glucoside | cZOG |
 | - | - | H | 6-benzylaminopurine | BAP |
 | - | - | H | meta-topolin | mT |
| - | - | ribosyl | meta-topolin riboside | mTR | |
 | - | - | H | ortho-topolin | oT |
| - | - | ribosyl | ortho-topolin riboside | oTR | |
 | - | - | H | para-topolin | pT |
| - | - | ribosyl | para-topolin riboside | pTR | |
 | - | - | H | kinetin | K |
| - | - | ribosyl | kinetin riboside | KR | |
| - | - | ribotide | KR-5‘-monophosphate | KMP | |
| glycosyl | - | - | kinetin-3-glucoside | K3G | |
| - | - | glycosyl | kinetin-9-glucoside | K9G | |
 | |||||
| Deferoxamine | DFO | ||||
 | N-acetylcysteine | NAC | |||
 | Necrostatin 1 | NEC-1 | |||
| Average (TE) | SD (n = 3) | Average (TE) | SD (n = 3) | ||
|---|---|---|---|---|---|
| tZ | 0.073 | 0.012 | K | 2.082 | 0.256 |
| tZR | 0.13 | 0.012 | KR | 1.673 | 0.294 |
| tZMP | 0.326 | 0.017 | KMP | 0.726 | 0.026 |
| tZ7G | 0.082 | 0.006 | K3G | 0.53 | 0.035 |
| tZ9G | 0.063 | 0.004 | K9G | 0.906 | 0.025 |
| tZOG | 0.042 | 0.006 | BAP | N/A * | N/A * |
| cZ | 0.151 | 0.009 | oT | 7.026 | 1.179 |
| cZR | 0.178 | 0.005 | oTR | 3.241 | 0.140 |
| cZMP | 0.173 | 0.036 | mT | 4.509 | 0.687 |
| cZ9G | 0.085 | 0.009 | mTR | 3.171 | 0.239 |
| cZOG | 3.301 | 0.036 | pT | 16.799 | 0.829 |
| iP | 0.384 | 0.005 | pTR | 4.147 | 0.238 |
| iPR | 0.224 | 0.024 |
| Compound | Viability a | Compound | Viability a |
|---|---|---|---|
| (%, 10 µM) | (%, 10 µM) | ||
| tZ | 102.3 ± 2.20 | K | 97.9 ± 1.81 |
| tZR | 98.8 ± 1.82 | KR | 88.1 ± 3.09 |
| tZMP | 101.5 ± 4.59 | KMP | 93.5 ± 3.57 |
| tZ7G | 101.4 ± 2.44 | K3G | 99.8 ± 1.13 |
| tZ9G | 97.6 ± 1.59 | K9G | 98.3 ± 1.11 |
| tZOG | 94.3 ± 1.67 | BAP | 98.8 ± 1.40 |
| cZ | 104.0 ± 1.79 | oT | 95.5 ± 4.02 |
| cZR | 100.0 ± 1.23 | oTR | 90.8 ± 3.81 |
| cZMP | 93.8 ± 2.13 | mT | 90.4 ± 6.03 |
| cZ9G | 101.7 ± 2.08 | mTR | 99.5 ± 3.69 |
| cZOG | 98.18 ± 1.59 | pT | 90.6 ± 3.25 |
| iP | 104.5 ± 0.97 | pTR | 89.5 ± 3.89 |
| iPR | 96.9 ± 3.25 | ||
| NAC (1 mM) | 90.2 ± 5.06 | DFO (100µM) | 102.1 ± 4.22 |
| NEC-1 (50 µM) | 95,8 ± 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, G.; Grúz, J.; D’Acunto, C.W.; Kaňovský, P.; Strnad, M. Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules 2021, 26, 361. https://doi.org/10.3390/molecules26020361
Gonzalez G, Grúz J, D’Acunto CW, Kaňovský P, Strnad M. Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules. 2021; 26(2):361. https://doi.org/10.3390/molecules26020361
Chicago/Turabian StyleGonzalez, Gabriel, Jiří Grúz, Cosimo Walter D’Acunto, Petr Kaňovský, and Miroslav Strnad. 2021. "Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease" Molecules 26, no. 2: 361. https://doi.org/10.3390/molecules26020361
APA StyleGonzalez, G., Grúz, J., D’Acunto, C. W., Kaňovský, P., & Strnad, M. (2021). Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson’s Disease. Molecules, 26(2), 361. https://doi.org/10.3390/molecules26020361