Methoxy-Substituted γ-Oxa-ε-Lactones Derived from Flavanones—Comparison of Their Anti-Tumor Activity In Vitro
Abstract
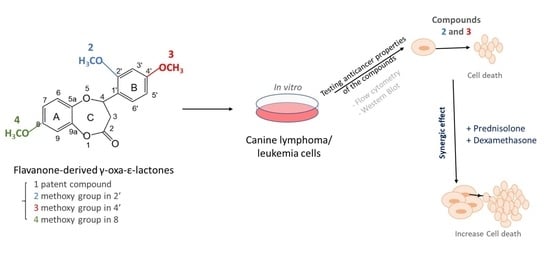
:1. Introduction
2. Results
2.1. Cell Viability Assay
2.2. Apoptosis Study
2.2.1. Annexin V/PI Staining
2.2.2. WB Analysis
2.3. Study of Synergistic Action of Flavanone-Derived γ-Oxa-ε-Lactones and Glucocorticoids
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Compound Preparation
4.3. Cell Lines and Cell Culture
4.4. Cell Viability Assay
4.5. Western Blotting
4.6. Flow Cytometry Apoptosis Assays
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Białońska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing...only different. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones derived from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Gładkowski, W.; Włoch, A.; Pawlak, A.; Sysak, A.; Białońska, A.; Mazur, M.; Mituła, P.; Maciejewska, G.; Obmińska-Mrukowicz, B.; Kleszczyńska, H. Preparation of Enantiomeric β-(2′,5′-Dimethylphenyl)Bromolactones, Their Antiproliferative Activity and Effect on Biological Membranes. Molecules 2018, 23, 3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlak, A.; Gładkowski, W.; Kutkowska, J.; Mazur, M.; Obmińska-Mrukowicz, B.; Rapak, A. Enantiomeric trans β-aryl-δ-iodo-γ-lactones derived from 2,5-dimethylbenzaldehyde induce apoptosis in canine lymphoma cell lines by downregulation of anti-apoptotic Bcl-2 family members Bcl-xL and Bcl-2. Bioorganic Med. Chem. Lett. 2018, 28, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Gładkowski, W.; Mazur, M.; Henklewska, M.; Obmińska-Mrukowicz, B.; Rapak, A. Optically active stereoisomers of 5-(1-iodoethyl)-4-(4′-isopropylphenyl)dihydrofuran-2-one: The effect of the configuration of stereocenters on apoptosis induction in canine cancer cell lines. Chem. Interactions 2017, 261, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Xu, H. Total synthesis of floresolide B and Δ6,7-Z-floresolide B. Chem. Commun. 2006, 600–602. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Cortese, M.; Saladino, R.; Gualandi, G.; Belfiore, M.C. Conversion of naringenin and hesperetin by heterogeneous catalytic Baeyer–Villiger reaction into lactones exhibiting apoptotic activity. Tetrahedron Lett. 2003, 44, 4823–4825. [Google Scholar] [CrossRef]
- Walle, T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Gładkowski, W.; Siepka, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Popłoński, J.; Mazur, M.; Żarowska, B.; Łaba, W.; Maciejewska, G.; Wawrzeńczyk, C. Synthesis and Antimicrobial Activity of Methoxy- Substituted γ-Oxa-ε-lactones Derived from Flavanones. Molecules 2019, 24, 4151. [Google Scholar] [CrossRef] [Green Version]
- Marconato, L.; Gelain, M.E.; Comazzi, S. The dog as a possible animal model for human non-Hodgkin lymphoma: A review. Hematol. Oncol. 2012, 31, 1–9. [Google Scholar] [CrossRef]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Buhrmann, C.; Varghese, E.; Abotaleb, M.; Qaradakhi, T.; Zulli, A.; et al. Flavonoids against the Warburg phenotype—concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020, 11, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech. 2013, 3, 439–459. [Google Scholar] [CrossRef] [Green Version]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Kozioł, A.; Mroczko, L.; Niewiadomska, M.; Lochyński, S. γ-lactones with potential biological activity. Pol. J. Natur. Sci. 2017, 32, 495–511. [Google Scholar]
- Gawdzik, B.; Kamizela, A.; Syszkowska, A. Laktony o właściwościach sensorycznych. Chemik 2015, 69, 342–349. [Google Scholar]
- Tsuji, P.A. Inhibition of benzo[a]pyrene-activating enzymes and DNA binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis 2005, 27, 1579–1585. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2017, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Zioło, E.; Kutkowska, J.; Blazejczyk, A.; Wietrzyk, J.; Krupa, A.; Hildebrand, W.; Dziegiel, P.; Dzimira, S.; Obminska-Mrukowicz, B.; et al. A novel canine B-cell leukaemia cell line. Establishment, characterisation and sensitivity to chemotherapeutics. Veter. Comp. Oncol. 2016, 15, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef]
- Nakaichi, M.; Taura, Y.; Kanki, M.; Mamba, K.; Momoi, Y.; Tsujimoto, H.; Nakama, S. Establishment and Characterization of a New Canine B-Cell Leukemia Cell Line. J. Veter. Med. Sci. 1996, 58, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Grudzien, M.; Pawlak, A.; Kutkowska, J.; Ziolo, E.; Wysokińska, E.; Hildebrand, W.; Obmińska-Mrukowicz, B.; Strzadala, L.; Rapak, A. A newly established canine NK-type cell line and its cytotoxic properties. Veter. Comp. Oncol. 2021, 19, 567–577. [Google Scholar] [CrossRef]
- Campbell, J.K.; King, J.L.; Harmston, M.; Lila, M.A.; Erdman, J.W. Synergistic Effects of Flavonoids on Cell Proliferation in Hepa-1c1c7 and LNCaP Cancer Cell Lines. J. Food Sci. 2006, 71, S358–S363. [Google Scholar] [CrossRef]
- Rauca, V.-F.; Vlase, L.; Casian, T.; Sesarman, A.; Gheldiu, A.-M.; Mocan, A.; Banciu, M.; Toiu, A.M. Biologically Active Ajuga Species Extracts Modulate Supportive Processes for Cancer Cell Development. Front. Pharmacol. 2019, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Ye, X.; Chen, Y.C. Flavonoids from Chinese bayberry leaves induced apoptosis and G1 cell cycle arrest via Erk pathway in ovarian cancer cells. Eur. J. Med. Chem. 2018, 147, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Sanchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S. Cancer phytotherapeutics: Role for flavonoids at the cellular level. Phytother. Res. 2008, 22, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.N.N.N.M.; Septama, A.W.; Simbak, N.; Abu Bakar, N.H.; Rahmi, E.P. Synergistic Effect of Flavonoids fromArtocarpus heterophyllusHeartwoods on Anticancer Activity of Cisplatin Against H460 and MCF-7 Cell Lines. Nat. Prod. Sci. 2019, 25, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.; Elbaz, H.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2015, 37, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]







| Compound/Cell Line | CLB-70 | CLBL-1 | CNK-89 | GL-1 |
|---|---|---|---|---|
| 1 | 26.13 ± 3.55 a | 19.96 ± 1.05 b | N.A. | 37.52 ± 1.27 c |
| 2 | 23.24 ± 2.63 a | 17.14 ± 0.59 a | N.A. | 36.41 ± 4.41 b |
| 3 | 8.25 ± 1.14 a | 7.99 ± 0.19 a | 31.52 ± 3.18 b | 18.95 ± 0.47 c |
| 4 | 31.94 ± 7.88 a | 31.49 ± 4.21 a | N.A. | 42.81 ± 0.07 a |
| Etoposide | 14.33 ± 2.12 | 0.02 ± 0.00 | N.I. | 4.43 ± 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak, A.; Henklewska, M.; Hernández-Suárez, B.; Siepka, M.; Gładkowski, W.; Wawrzeńczyk, C.; Motykiewicz-Pers, K.; Obmińska-Mrukowicz, B. Methoxy-Substituted γ-Oxa-ε-Lactones Derived from Flavanones—Comparison of Their Anti-Tumor Activity In Vitro. Molecules 2021, 26, 6295. https://doi.org/10.3390/molecules26206295
Pawlak A, Henklewska M, Hernández-Suárez B, Siepka M, Gładkowski W, Wawrzeńczyk C, Motykiewicz-Pers K, Obmińska-Mrukowicz B. Methoxy-Substituted γ-Oxa-ε-Lactones Derived from Flavanones—Comparison of Their Anti-Tumor Activity In Vitro. Molecules. 2021; 26(20):6295. https://doi.org/10.3390/molecules26206295
Chicago/Turabian StylePawlak, Aleksandra, Marta Henklewska, Beatriz Hernández-Suárez, Monika Siepka, Witold Gładkowski, Czesław Wawrzeńczyk, Karolina Motykiewicz-Pers, and Bożena Obmińska-Mrukowicz. 2021. "Methoxy-Substituted γ-Oxa-ε-Lactones Derived from Flavanones—Comparison of Their Anti-Tumor Activity In Vitro" Molecules 26, no. 20: 6295. https://doi.org/10.3390/molecules26206295
APA StylePawlak, A., Henklewska, M., Hernández-Suárez, B., Siepka, M., Gładkowski, W., Wawrzeńczyk, C., Motykiewicz-Pers, K., & Obmińska-Mrukowicz, B. (2021). Methoxy-Substituted γ-Oxa-ε-Lactones Derived from Flavanones—Comparison of Their Anti-Tumor Activity In Vitro. Molecules, 26(20), 6295. https://doi.org/10.3390/molecules26206295