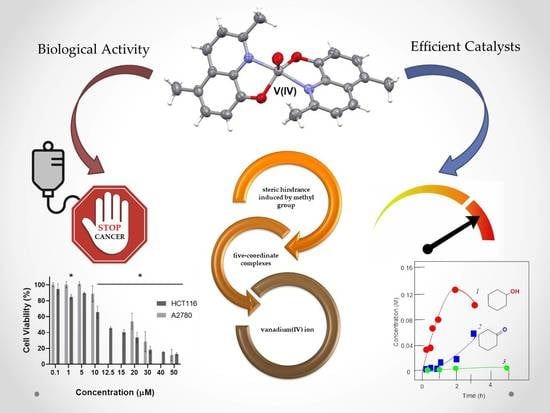
Vanadium(IV) Complexes with Methyl-Substituted 8-Hydroxyquinolines: Catalytic Potential in the Oxidation of Hydrocarbons and Alcohols with Peroxides and Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Molecular Structure
2.3. EPR Spectroscopy
2.4. Absorption Spectroscopy
2.5. Catalytic Oxidations with Hydrogen Peroxide
2.6. Viability Studies
2.7. Complex Internalization
2.8. Induction of Apoptosis in the HCT116 Cell Line Exposed to Complexes 1–3
2.9. Induction of Autophagy in the HCT116 Cell Line Exposed to Complexes 1–3
2.10. Intracellular Reactive Oxygen Species (ROS) Production in the HCT116 Cell Line Exposed to Complexes 1–3
2.11. Evaluation of Alterations in the Mitochondrial Membrane Potential of HCT116 Cells Exposed to Complexes 1–3
3. Materials and Methods
3.1. Materials
3.2. Synthesis of [VO(2,6-(Me)2-quin)2] (1) and [VO(2,5-(Me)2-quin)2] (2)
3.3. X-ray Crystal Structure Determination
3.4. Physical Measurements
3.5. EPR Spectroscopy
3.6. Biological Assays
3.6.1. Cell Culture
3.6.2. Viability Assays
3.6.3. Vanadium Detection in the HCT116 Cell Line by ICP-AES
3.6.4. Evaluation of Apoptosis Induction in the HCT116 Cell Line by Flow Cytometry
3.6.5. Autophagy Induction Evaluation in the HCT116 Cell Line by Flow Cytometry
3.6.6. Intracellular Reactive Oxygen Species (ROS) Production Evaluation in the HCT116 Cell Line by Flow Cytometry
3.6.7. Mitochondrial Membrane Potential Evaluation in the HCT116 Cell Line by Flow Cytometry
3.7. Catalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Butler, A.; Carrano, C. Coordination chemistry of vanadium in biological systems. Coord. Chem. Rev. 1991, 109, 61–105. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ghosh, S.; Wu, B.-M.; Mak, T.C.W. A structural and electrochemical study of some oxovanadium (IV) heterochelate complexes. Polyhedron 1998, 17, 1369–1374. [Google Scholar] [CrossRef]
- Liu, S.-X.; Gao, S. Synthesis, crystal structure and spectral properties of VO (acetylacetone benzoylhydrazone) (8-quinolinol). Polyhedron 1998, 17, 81–84. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mukhopadhyay, S.; Samanta, S.; Weakley, T.J.R.; Chaudhury, M. Synthesis, characterization, and reactivity of mononuclear O, N-chelated vanadium(IV) and -(III) complexes of methyl 2-aminocyclopent-1-ene-1-dithiocarboxylate based ligand: Reporting an example of conformational isomerism in the solid state. Inorg. Chem. 2002, 41, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Horn, A., Jr.; Filgueiras, C.A.L.; Wardell, J.L.; Herbst, M.H.; Vugman, N.V.; Santos, P.S.; Lopes, J.G.S.; Howie, R.A. A fresh look into VO (salen) chemistry: Synthesis, spectroscopy, electrochemistry and crystal structure of [VO(salen)(H2O)]Br·0.5CH3CN. Inorg. Chim. Acta 2004, 357, 4240–4246. [Google Scholar] [CrossRef]
- Elias, H.; Schwartze-Eidam, S.; Wannowius, K.J. Kinetics and mechanism of ligand substitution in bis(N-alkylsalicylaldiminato)oxovanadium(IV) complexes. Inorg. Chem. 2003, 42, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Pessoa, J.C.; Duarte, M.T.; Henriques, R.T.; Fátima, M.; Piedade, M.; Veiros, L.F.; Jakusch, T.; Kiss, T.; Dörnyei, Á.; et al. N,N′-ethylenebis(pyridoxylideneiminato) and N,N′-ethylenebis(pyridoxylaminato): Synthesis, characterization, potentiometric, spectroscopic, and DFT studies of their vanadium(IV) and vanadium(V) complexes. Chem. Eur. J. 2004, 10, 2301–2317. [Google Scholar] [CrossRef] [Green Version]
- Yucesan, G.; Armatas, N.G.; Zubieta, J. Hydrothermal synthesis of molecular oxovanadium compounds. The crystal and molecular structures of [VO2(terpy)]NO3, [VO(terpy)(OH3PC6H5)2], [{Cu(H2O)(terpy)}V2O6], [{Cu(ttbterpy)}V2O6] and [{Cu(ttbterpy)}VO2(HO3PCH2PO3)]·H2O (terpy = 2,2′:6′,2″-terpyridine; ttbterpy = 4,4′,4″-tri-tert-butyl-2,2′:6′,2″-terpyridine). Inorg. Chim. Acta 2006, 359, 4557–4564. [Google Scholar]
- Ghosh, T.; Mondal, B.; Ghosh, T.; Sutradhar, M.; Mukherjee, G.; Drew, M.G.B. Synthesis, structure, solution chemistry and the electronic effect of para substituents on the vanadium center in a family of mixed-ligand [VVO(ONO)(ON)] complexes. Inorg. Chim. Acta 2007, 360, 1753–1761. [Google Scholar] [CrossRef]
- Rubčić, M.; Milić, D.; Horvat, G.; Ðilović, I.; Galić, N.; Tomšić, V.; Cindrić, M. Vanadium-induced formation of thiadiazole and thiazoline compounds. Mononuclear and dinuclear oxovanadium(V) complexes with open-chain and cyclized thiosemicarbazone ligands. Dalton Trans. 2009, 2009, 9914–9923. [Google Scholar] [CrossRef]
- Hakimi, M.; Kukovec, B.-M.; Rezvaninezhad, M.; Schuh, E.; Mohr, F. Preparation, structural and spectroscopic characterization of vanadium(IV) and vanadium(V) complexes with dipicolinic acid. Z. Anorg. Allg. Chem. 2011, 637, 2157–2162. [Google Scholar] [CrossRef]
- Sanna, D.; Buglyó, P.; Tomaz, A.I.; Costa Pessoa, J.; Borović, S.; Micerae, G.; Garribba, E. VIVO and CuII complexation by ligands based on pyridine nitrogen donors. Dalton Trans. 2012, 41, 12824–12838. [Google Scholar] [CrossRef] [PubMed]
- Tutusaus, O.; Ni, C.; Szymczak, N.K. A Transition metal Lewis acid/base triad system for cooperative substrate binding. J. Am. Chem. Soc. 2013, 135, 3403–3406. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Maity, M.; Abtab, S.M.T.; Majee, M.C.; Chaudhury, M. Homo- and heterometal complexes of oxido–metal ions with a triangular [V(V)O–MO–V(V)O] [M = V(IV) and Re(V)] core: Reporting mixed-oxidation oxido–vanadium(V/IV/V) compounds with valence trapped structures. Inorg. Chem. 2013, 52, 9597–9605. [Google Scholar] [CrossRef]
- Sheng, G.-H.; Cheng, X.-S.; You, Z.-L.; Zhu, H.-L. Two isomeric structures of oxovanadium(V) complexes with hydrazone and 8-hydroxyquinoline ligands. J. Struct. Chem. 2015, 56, 942–947. [Google Scholar] [CrossRef]
- Sheppard, B.J.H.; Shaver, M.P.; Pearson, J.K. Assessment and application of density functional theory for the prediction of structure and reactivity of vanadium complexes. J. Phys. Chem. A 2015, 119, 8537–8546. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Pombeiro, A.J.L. Coordination chemistry of non-oxido, oxido and dioxidovanadium(IV/V) complexes with azine fragment ligands. Coord. Chem. Rev. 2014, 265, 89–124. [Google Scholar] [CrossRef]
- Kolesa-Dobravc, T.; Lodyga-Chruscinska, E.; Symonowicz, M.; Sanna, D.; Meden, A.; Perdih, F.; Garribba, E. Synthesis and characterization of V(IV)O complexes of picolinate and pyrazine derivatives. Behavior in the solid state and aqueous solution and biotransformation in the presence of blood plasma proteins. Inorg. Chem. 2014, 53, 7960–7976. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Furness, L.; Levason, W.; Reid, G.; Zhang, W. Complexes of vanadium(IV) oxide difluoride with neutral N- and O-donor ligands. J. Fluor. Chem. 2016, 191, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.P.; Majumder, S.; Banerjee, A.; Fernanda, M.; Carvalho, N.N.; Adaão, P.; Costa Pessoa, J.; Brzezinski, K.; Garribba, E.; Reuter, H.; et al. Chemistry of monomeric and dinuclear non-oxido vanadium(IV) and oxidovanadium(V) aroylazine complexes: Exploring solution behavior. Inorg. Chem. 2016, 55, 1165–1182. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Gonzalez Cuervo, L.; Therrien, B.; Stoeckli-Evans, H.; Shul’pin, G.B. Mono and oligonuclear vanadium complexes as catalysts for alkane oxidation: Synthesis, molecular structure, and catalytic potential. Inorg. Chim. Acta 2004, 357, 475–484. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.L.; Silks, L.A.; Thorn, D.L. Mechanism of alcohol oxidation by dipicolinate vanadium (V): Unexpected role of pyridine. J. Am. Chem. Soc. 2010, 132, 17804–17816. [Google Scholar] [CrossRef]
- Zhang, G.; Scott, B.L.; Wu, R.; Silks, L.A.; Hanson, S.K. Aerobic oxidation reactions catalyzed by vanadium complexes of bis (phenolate) ligands. Inorg. Chem. 2012, 51, 7354–7361. [Google Scholar] [CrossRef]
- Grivani, G.; Ghavami, A.; Kučeráková, M.; Dušek, M.; Dehno Khalaji, A. Synthesis, characterization, crystal structure determination, thermal study and catalytic activity of a new oxidovanadium Schiff base complex. J. Mol. Struct. 2014, 1076, 326–332. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Kolosov, N.A.; Kurmaev, D.A.; Gagieva, S.C.; Khrustalev, V.N.; Ikonnikov, N.S.; Efimov, N.N.; Ugolkova, E.A.; Minin, V.V.; Bulychev, B.M. Vanadium (IV), (V) coordination compounds with 8-hydroxyquinoline derivative: Synthesis, structure and catalytic activity in the polymerization of ethylene. J. Organomet. Chem. 2015, 798, 393–400. [Google Scholar] [CrossRef]
- Mandal, M.; Nagaraju, V.; Karunakar, G.V.; Sarma, B.; Borah, B.J.; Bania, K.K. Electronic, conjugation, and confinement effects on structure, redox, and catalytic behavior of oxido-vanadium (IV) and-(V) chiral Schiff base complexes. J. Phys. Chem. C 2015, 119, 28854–28870. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Chieregato, A.; Lopez Nieto, J.M.; Cavani, F. Mixed-oxide catalysts with vanadium as the key element for gas-phase reactions. Coord. Chem. Rev. 2015, 301–302, 3–23. [Google Scholar] [CrossRef]
- Elkurtehi, A.I.; Walsh, A.G.; Dawe, L.N.; Kerton, F.M. Vanadium Aminophenolate complexes and their catalytic activity in aerobic and H2O2-mediated oxidation reactions. Eur. J. Inorg. Chem. 2016, 2016, 3123–3130. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.-M.; Bai, F.-Y.; Sun, L.-X. Novel vanadium complexes with rigid carboxylate ligands: Synthesis, structure and catalytic bromine dynamics of phenol red. J. Mol. Struct. 2017, 1149, 379–386. [Google Scholar] [CrossRef]
- Schmidt, A.-C.; Hermsen, M.; Rominger, F.; Dehn, R.; Teles, J.H.; Schaäfer, A.; Trapp, O.; Schaub, T. Synthesis of mono-and dinuclear vanadium complexes and their reactivity toward dehydroperoxidation of alkyl hydroperoxides. Inorg. Chem. 2017, 56, 1319–1332. [Google Scholar] [CrossRef]
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The coordination chemistry of vanadium as related to its biological functions. Coord. Chem. Rev. 1999, 182, 297–322. [Google Scholar] [CrossRef]
- Xie, M.-J.; Niu, Y.-F.; Yang, X.-D.; Liu, W.-P.; Li, L.; Gao, L.-H.; Yan, S.-P.; Meng, Z.-H. Effect of the chloro-substitution on lowering diabetic hyperglycemia of vanadium complexes with their permeability and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 6077–6084. [Google Scholar] [CrossRef]
- Banik, B.; Sasmal, P.K.; Roy, S.; Majumdar, R.; Dighe, R.R.; Chakravarty, A.R. Terpyridine oxovanadium (IV) complexes of phenanthroline bases for cellular imaging and photocytotoxicity in HeLa cells. Eur. J. Inorg. Chem. 2011, 2011, 1425–1435. [Google Scholar] [CrossRef]
- Correia, I.; Adão, P.; Roy, S.; Wahba, M.; Matos, C.; Maurya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.F.; Avecilla, F.; et al. Hydroxyquinoline derived vanadium(IV and V) and copper(II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar] [CrossRef]
- Fik, M.A.; Gorczyński, A.; Kubicki, M.; Hnatejko, Z.; Wadas, A.; Kulesza, P.J.; Lewińska, A.; Giel-Pietraszuk, M.; Wyszko, E.; Patroniak, V. New vanadium complexes with 6,6″-dimethyl-2,2′:6′,2″-terpyridine in terms of structure and biological properties. Polyhedron 2015, 97, 83–93. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Reytman, L.; Braitbard, O.; Hochman, J.; Tshuva, E.Y. Highly effective and hydrolytically stable vanadium (V) amino phenolato antitumor agents. Inorg. Chem. 2016, 55, 610–618. [Google Scholar] [CrossRef]
- Kumar, A.; Pant, I.; Dixit, A.; Banerjee, S.; Banik, B.; Saha, R.; Kondaiah, P.; Chakravarty, A.R. Terpyridyl oxovanadium (IV) complexes for DNA crosslinking and mito-targeted photocytotoxicity. J. Inorg. Biochem. 2017, 174, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.-L.; Liu, L.-J.; Lu, W.-G.; Wang, X.-B. A vanadium (V) terpyridine complex: Synthesis, characterization, cytotoxicity in vitro and induction of apoptosis in cancer cells. Transit. Met. Chem. 2017, 42, 459–467. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, H.; Tao, L.; Li, X.; Zhou, Z.; Sun, Y.; Chen, C.; Wei, D.; Liu, Y.; Diao, G. Synthesis, in vitro cytotoxicity, and structure–activity relationships (SAR) of multidentate oxidovanadium(IV) complexes as anticancer agents. Dalton Trans. 2018, 47, 10035–10045. [Google Scholar] [CrossRef]
- El-Deen, I.M.; Shoair, A.F.; El-Bindary, M.A. Synthesis, characterization and biological properties of oxovanadium(IV) complexes. J. Mol. Struct. 2019, 1180, 420–437. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable vanadium-catalyzed oxidation of organic substrates with H2O2. Catal. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Idouhli, R.; Nayad, A.; Ouahine, H.; Khadiri, M.-E.; Abouelfidab, A.; Elfirdoussi, L.; Ait Ali, M. Di-nuclear water-soluble oxovanadium (V) Schiff base complexes: Electrochemical properties and catalytic oxidation. Inorg. Chem. Commun. 2020, 119, 108134. [Google Scholar]
- Crans, D. Chemistry and insulin-like properties of vanadium (IV) and vanadium (V) compounds. J. Inorg. Biochem. 2000, 80, 123–131. [Google Scholar] [CrossRef]
- Banik, B.; Somyajit, K.; Koleyc, D.; Nagaraju, G.; Chakravarty, A.R. Cellular uptake and remarkable photocytotoxicity of pyrenylter pyridine oxovanadium(IV) complexes of dipyridophenazine bases. Inorg. Chim. Acta 2012, 393, 284–293. [Google Scholar] [CrossRef]
- Banik, B.; Somyajit, K.; Nagaraju, G.; Chakravarty, A.R. Oxovanadium (IV) catecholates of terpyridine bases for cellular imaging and photocytotoxicity in red light. RSC Adv. 2014, 4, 40120–40131. [Google Scholar] [CrossRef]
- Banik, B.; Somyajit, K.; Nagaraju, G.; Chakravarty, A.R. Oxovanadium (IV) complexes of curcumin for cellular imaging and mitochondria targeted photocytotoxicity. Dalton Trans. 2014, 43, 13358–13369. [Google Scholar] [CrossRef]
- Banik, B.; Somyajit, K.; Hussain, A.; Nagaraju, G.; Chakravarty, A.R. Carbohydrate-appended photocytotoxic (imidazophenanthroline)-oxovanadium (IV) complexes for cellular targeting and imaging. Dalton Trans. 2014, 43, 1321–1331. [Google Scholar] [CrossRef]
- Balaji, B.; Balakrishnan, B.; Perumalla, S.; Karande, A.A.; Chakravarty, A.R. Photoactivated cytotoxicity of ferrocenyl-terpyridine oxovanadium(IV) complexes of curcuminoids. Eur. J. Med. Chem. 2014, 85, 458–467. [Google Scholar] [CrossRef]
- Balaji, B.; Balakrishnan, B.; Perumalla, S.; Karande, A.A.; Chakravarty, A.R. Photocytotoxic oxovanadium(IV) complexes of ferrocenyl-terpyridine and acetylacetonate derivatives. Eur. J. Med. Chem. 2015, 92, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; McLeod, A.I.; Gasparini, S.J.; Nguyen, A.; Manori De Silva, W.G.; Aitken, J.B.; Harris, H.H.; Glover, C.; Johannessen, B.; Lay, P.A. Reactivity and speciation of anti-diabetic vanadium complexes in whole blood and its components: The important role of red blood cells. Inorg. Chem. 2015, 54, 7753–7766. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Fujisawa, Y.; Ohya-Nishiguchi, H.; Kamada, H.; Sakurai, H. In vivo coordination structural changes of a potent insulin-mimetic agent, bis(picolinato)oxovanadium(IV), studied by electron spin-echo envelope modulation spectroscopy. J. Inorg. Biochem. 1999, 77, 215–224. [Google Scholar] [CrossRef]
- Thompson, K.H.; McNeill, J.H.; Orvig, C. Vanadium compounds as insulin mimics. Chem. Rev. 1999, 99, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Orvig, C. Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord. Chem. Rev. 2001, 219–221, 1033–1053. [Google Scholar] [CrossRef]
- Sakurai, H.; Kojima, M.; Yoshikawa, Y.; Kawabe, K.; Yasui, H. Antidiabetic vanadium (IV) and zinc (II) complexes. Coord. Chem. Rev. 2002, 226, 187–198. [Google Scholar] [CrossRef]
- Kiss, T.; Jakusch, T.; Hollender, D.; Enyedy, EÉA.; Horvath, L. Comparative studies on the biospeciation of antidiabetic VO(IV) and Zn(II) complexes. J. Inorg. Biochem. 2009, 103, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Raposo, L.R.; Palion-Gazda, J.; Malicka, E.; Erfurt, K.; Machura, B.; Fernandes, A.R. In vitro antiproliferative effect of vanadium complexes bearing 8-hydroxyquinoline-based ligands–the substituent effect. Dalton Trans. 2020, 49, 6596–6606. [Google Scholar] [CrossRef] [PubMed]
- Gryca, I.; Czerwińska, K.; Machura, B.; Chrobok, A.; Shul’pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High catalytic activity of vanadium complexes in alkane oxidations with hydrogen peroxide: An effect of 8-hydroxyquinoline derivatives as noninnocent ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Shiro, M.; Fernando, Q. Structures of two five-coordinated metal chelates of 2-methyl-8-quinolinol. Anal. Chem. 1971, 43, 1222–1230. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands—The crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1984, 1349–1356. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Menati, S.; Rudbari, H.A.; Khorshidifard, M.; Jalilian, F. A new oxovanadium(IV) complex containing an O,N-bidentate Schiff base ligand: Synthesis at ambient temperature, characterization, crystal structure and catalytic performance in selective oxidation of sulfides to sulfones using H2O2 under solvent-free conditions. J. Mol. Struct. 2016, 1103, 94–102. [Google Scholar]
- Grivani, G.; Khalaji, A.D.; Tahmasebi, V.; Gotoh, K.; Ishida, H. Synthesis, characterization and crystal structures of new bidentate Schiff base ligand and its vanadium(IV) complex: The catalytic activity of vanadyl complex in epoxidation of alkenes. Polyhedron 2012, 31, 265–271. [Google Scholar] [CrossRef]
- Burgess, J.; Fawcett, J.; Palma, V.; Gilani, S.R. Fluoro derivatives of bis (salicylideneaminato-N, O) copper(II) and-oxovanadium(IV). Acta Crystallogr. Sect. C Struct. Sci. Cryst. Eng. Mater. 2001, 57, 277–280. [Google Scholar] [CrossRef]
- Santoni, G.; Rehder, D. Structural models for the reduced form of vanadate-dependent peroxidases: Vanadyl complexes with bidentate chiral Schiff base ligands. J. Inorg. Biochem. 2004, 98, 758–764. [Google Scholar] [CrossRef]
- Cornman, C.R.; Geiser-Bush, K.M.; Rowley, S.P.; Boyle, P.D. Structural and electron paramagnetic resonance studies of the square pyramidal to trigonal bipyramidal distortion of vanadyl complexes containing sterically crowded schiff base ligands. Inorg. Chem. 1997, 36, 6401–6408. [Google Scholar] [CrossRef]
- Pasquali, M.; Marchetti, F.; Floriani, C.; Merlino, S. Oxovanadium(IV) complexes containing bidentate Schiff-base ligands: Synthesis and structural and spectroscopic data. J. Chem. Soc. Dalton Trans. 1977, 1977, 139–144. [Google Scholar] [CrossRef]
- Cashin, B.; Cunningham, D.; Daly, P.; McArdle, P.; Munroe, M.; Chonchubhair, N.N. Donor properties of the vanadyl ion: Reactions of vanadyl salicylaldimine β-ketimine and acetylacetonato complexes with groups 14 and 15 Lewis acids. Inorg. Chem. 2002, 41, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, R.E.; Hughes, J.E.; Miller, T.H.; Shaikh, N.; Cunningham, P.H.M.; O’Connor, A.E.; Tidey, J.P.; Blake, A.J. Crystal structure of {2,2′-[ethylenebis(nitrilomethanylylidene)]diphenolato-/4O,N,N′,O′}oxidovanadium(IV) methanol monosolvate. Acta Crystallogr. Sect. E Struct. Sci. Cryst. Eng. Mater 2014, 70, m380–m381. [Google Scholar]
- Nguyen, M.T.; Jones, R.A.; Holliday, B.J. Effect of conjugation length and metal-backbone interactions on charge transport properties of conducting metallopolymers. Polym. Chem. 2017, 8, 4359–4367. [Google Scholar] [CrossRef]
- Carter, E.; Fallis, I.A.; Kariuki, B.M.; Morgan, I.R.; Murphy, D.M.; Tatchell, T.; Van Doorslaer, S.; Vinck, E. Structure and pulsed EPR characterization of N,N′-bis(5-tert-butylsalicylidene)-1,2-cyclohexanediamino-vanadium(IV) oxide and its adducts with propylene oxide. Dalton Trans. 2011, 40, 7454–7462. [Google Scholar] [CrossRef]
- Hoshina, G.; Tsuchimoto, M.; Ohba, S.; Nakajima, K.; Uekusa, H.; Ohashi, Y.; Ishida, H.; Kojima, M. Thermal Dehydrogenation of Oxovanadium(IV) Complexes with Schiff Base Ligands Derived from meso-1,2-Diphenyl-1,2-ethanediamine in the Solid State. Inorg. Chem. 1998, 37, 142–145. [Google Scholar] [CrossRef]
- Hoshina, G.; Tsuchimoto, M.; Ohba, S. exo-[(RS,SR)-N,N′-Bis(salicylidene)-2,3-butanediaminato]oxovanadium(IV). Acta Crystallogr. Sect. C Struct. Sci. Cryst. Eng. Mater. 1999, 55, 1082–1084. [Google Scholar] [CrossRef]
- Bonadies, J.A.; Butler, W.M.; Pecoraro, V.L.; Carrano, C.J. Novel reactivity patterns of (N,N′-ethylenebis(salicylideneaminato))oxovanadium(IV) in strongly acidic media. Inorg. Chem. 1987, 26, 1218–1222. [Google Scholar] [CrossRef]
- Hoshina, G.; Tsuchimoto, M.; Ohba, S. endo-{6,6′-Diethoxy-2,2′-[(R)-propane-1,2-diylbis(nitrilomethylidene)]diphenolato-O,N,N′,O′}oxovanadium(IV). Acta Crystallogr. Sect. C Struct. Sci. Cryst. Eng. Mater. 1999, 55, 1812–1813. [Google Scholar] [CrossRef]
- Bolm, C.; Bienewald, F.; Harms, K. Syntheses and vanadium complex of salen-like bissulfoximines. Synlett 1996, 8, 775–776. [Google Scholar] [CrossRef]
- Oyaizu, K.; Dewi, E.L.; Tsuchida, E. Coordination of BF4- to Oxovanadium(V) Complexes, Evidenced by the Redox Potential of Oxovanadium(IV/V) Couples in CH2Cl2. Inorg. Chem. 2003, 42, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Chasteen, N.D. Biological Magnetic Resonance; Berliner, L.J., Reuben, J., Eds.; Plenum: New York, NY, USA, 1981; Volume 3, pp. 53–119. [Google Scholar]
- Velayutham, M.; Varghese, B.; Subramanian, S. Magneto–structural correlation studies of a ferromagnetically coupled dinuclear vanadium(IV) complex. Single-crystal EPR study. Inorg. Chem. 1998, 37, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Shulpin, B.; Attanasio, D.; Suber, L. Efficient H2O2 oxidation of alkanes and arenes to alkyl peroxides and phenols catalyzed by the system vanadate-pyrazine-2-carboxylic acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’pin, G.B. Oxidation of CH compounds with peroxides catalyzed by polynuclear transition metal complexes in Si-or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Shul’pin, B.; Shul’pina, L.S. Vanadium Catalysis; Sutradhar, M., da Silva, J.A.L., Pombeiro, A.J.L., Eds.; Royal Society of Chemistry: London, UK, 2020; Chapter 4; pp. 72–96. [Google Scholar]
- Shul’pin, G.B.; Süss-Fink, G. Oxidations by the reagent “H2O2–vanadium complex–pyrazine-2-carboxylic acid”. Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O2 with urea. J. Chem. Soc. Perkin Trans. 2 1995, 7, 1459–1463. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Drago, R.S.; Gonzalez, M. Oxidations by a “H2O2–vanadium complex–pyrazine-2-carboxylic acid” reagent. Part 5. Oxidation of lower alkanes with the formation of carbonyl compounds”. Russ. Chem. Bull. 1996, 45, 2386–2388. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Guerreiro, M.C.; Schuchardt, U. Oxidations by the reagent O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid. Part 7. Hydroperoxidation of higher alkanes. Tetrahedron 1996, 52, 13051–13062. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N.; Nizova, G.V. Oxidation with the H2O2–VO3––pyrazine-2-carboxylic acid reagent. Part 2. Oxidation of alcohols and aromatic hydrocarbons. Russ. Chem. Bull. 1993, 42, 1327–1329. [Google Scholar]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Ertik, O.; Kalındemirtaş, F.D.; Kaya, B.; Yanardag, R.; Erdem Kuruca, S.; Şahin, O.; Ülküseven, B. Oxovanadium(IV) complexes with tetradentate thiosemicarbazones. Synthesis, characterization, anticancer enzyme inhibition and in vitro cytotoxicity on breast cancer cells. Polyhedron 2021, 202, 115192. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.; Ferretti, F.; Raposo, L.R.; Guedes da Silva, M.F.C.; Baptista, P.V.; Fernandes, A.R.; Pombeiro, A.J.L. Antiproliferative activity of heterometallic sodium and potassium-dioxidovanadium (V) polymers. J. Inorg. Biochem. 2019, 200, 110811. [Google Scholar] [CrossRef]
- Yang, X.G.; Wang, K.; Lu, J.F.; Crans, D.C. Membrane transport of vanadium compounds and the interaction with the erythrocyte membrane. Coord. Chem. Rev. 2003, 237, 103–111. [Google Scholar] [CrossRef]
- Reigosa-Chamorro, F.; Raposo, L.R.; Munin-Cruz, P.; Pereira, M.T.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R.; Vila, J.M. In Vitro and In Vivo Effect of Palladacycles: Targeting A2780 Ovarian Carcinoma Cells and Modulation of Angiogenesis. Inorg. Chem. 2021, 60, 3939–3951. [Google Scholar] [CrossRef] [PubMed]
- Nycz, J.E.; Szala, M.; Malecki, G.J.; Nowak, M.; Kusz, J. Synthesis, spectroscopy and computational studies of selected hydroxyquinolines and their analogues. Spectrochim. Acta Part A 2014, 117, 351. [Google Scholar] [CrossRef]
- CrysAlisPRO; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Sci. Cryst. Eng. Mater. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Várnagy, K.; Lihi, N.; Micera, G.; Garribba, E. Formation of new non-oxido vanadium(IV) species in aqueous solution and in the solid state by tridentate (O, N, O) ligands and rationalization of their EPR behavior. Inorg. Chem. 2013, 52, 8202–8213. [Google Scholar] [CrossRef]
- WINEPR SimFonia; Version 1.25; Bruker Analytische, Messtechnik GmbH: Karlshruhe, Germany, 1996.
- Raposo, L.R.; Silva, A.; Silva, D.; Roma-Rodrigues, C.; Espadinha, M.; Baptista, P.V.; Santos, M.M.M.; Fernandes, A.R. Exploiting the antiproliferative potential of spiropyrazoline oxindoles in a human ovarian cancer cell line. Bioorg. Med. Chem. 2021, 30, 115880. [Google Scholar] [CrossRef]
- Choroba, K.; Machura, B.; Szlapa-Kula, A.; Malecki, J.G.; Raposo, L.; Roma-Rodrigues, C.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Square planar Au (III), Pt (II) and Cu (II) complexes with quinoline-substituted 2, 2′: 6′, 2″-terpyridine ligands: From in vitro to in vivo biological properties. Eur. J. Med. Chem. 2021, 218, 113404. [Google Scholar] [CrossRef] [PubMed]
- Reers, M.; Smith, T.W.; Chen, L.B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(µ-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
















| Cell Lines | 1 (µM) | 2 (µM) | 3 (µM) |
|---|---|---|---|
| HCT116 | 12.5 ± 0.63 | 9.6 ± 0.54 | 5.9 ± 0.66 |
| A2780 | 17.5 ± 1.13 | 20.7 ± 3.11 | 50.9 ± 2.75 |
| Fibroblasts | 15.8 ± 1.49 | 8.1 ± 2.75 | 14.5 ± 2.33 |
| 1 | 2 | |
|---|---|---|
| Empirical formula | C22H20N2O3V | C22H20N2O3V |
| Formula weight | 411.34 | 411.34 |
| T, K | 295.0(2) | 295.0(2) |
| Wavelength, Å | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/c |
| Unit cell dimensions, Å and ° | ||
| a | 18.8566(15) | 15.6046(11) |
| b | 8.2237(4) | 8.1475(5) |
| c | 13.2105(8) | 16.5949(16) |
| β | 113.906(5) | 117.340(11) |
| V, Å3 | 1872.8(2) | 1874.2(3) |
| Z | 4 | 4 |
| Dc, g cm−3 | 1.459 | 1.458 |
| Absorption coefficient, mm−1 | 0.556 | 0.555 |
| F(000) | 852 | 852 |
| Crystal size, mm | 0.283 × 0.137 × 0.055 | 0.162 × 0.089 × 0.079 |
| θ range for data collection ° | 3.46 to 25.05 | 3.49 to 25.05 |
| Index ranges | −22 ≤ h ≤ 22 −9 ≤ k ≤ 9 −15 ≤ l ≤ 15 | −18 ≤ h ≤ 17 −8 ≤ k ≤ 9 −19 ≤ l ≤ 18 |
| Reflections collected | 6154 | 7065 |
| Independent reflections | 1653 [Rint = 0.0221] | 3291 [Rint = 0.0479] |
| Completeness to 2θ | 99.7 | 99.3 |
| Min. and max. transm. | 0.712 and 1.000 | 0.588 and 1.000 |
| Data/restraints/parameters | 1653/0/130 | 3291/0/257 |
| Goodness-of-fit on F2 | 1.086 | 1.002 |
| Final R indices [I > 2σ(I)] R1 wR2 | 0.0326 0.0931 | 0.0489 0.1065 |
| R indices (all data) R1 wR2 | 0.0355 0.0950 | 0.0834 0.1202 |
| Largest diff. peak and hole, e Å−3 | 0.50 and −0.24 | 0.34 and −0.32 |
| CCDC number | 1971585 | 1971586 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palion-Gazda, J.; Luz, A.; Raposo, L.R.; Choroba, K.; Nycz, J.E.; Bieńko, A.; Lewińska, A.; Erfurt, K.; V. Baptista, P.; Machura, B.; et al. Vanadium(IV) Complexes with Methyl-Substituted 8-Hydroxyquinolines: Catalytic Potential in the Oxidation of Hydrocarbons and Alcohols with Peroxides and Biological Activity. Molecules 2021, 26, 6364. https://doi.org/10.3390/molecules26216364
Palion-Gazda J, Luz A, Raposo LR, Choroba K, Nycz JE, Bieńko A, Lewińska A, Erfurt K, V. Baptista P, Machura B, et al. Vanadium(IV) Complexes with Methyl-Substituted 8-Hydroxyquinolines: Catalytic Potential in the Oxidation of Hydrocarbons and Alcohols with Peroxides and Biological Activity. Molecules. 2021; 26(21):6364. https://doi.org/10.3390/molecules26216364
Chicago/Turabian StylePalion-Gazda, Joanna, André Luz, Luis R. Raposo, Katarzyna Choroba, Jacek E. Nycz, Alina Bieńko, Agnieszka Lewińska, Karol Erfurt, Pedro V. Baptista, Barbara Machura, and et al. 2021. "Vanadium(IV) Complexes with Methyl-Substituted 8-Hydroxyquinolines: Catalytic Potential in the Oxidation of Hydrocarbons and Alcohols with Peroxides and Biological Activity" Molecules 26, no. 21: 6364. https://doi.org/10.3390/molecules26216364
APA StylePalion-Gazda, J., Luz, A., Raposo, L. R., Choroba, K., Nycz, J. E., Bieńko, A., Lewińska, A., Erfurt, K., V. Baptista, P., Machura, B., Fernandes, A. R., Shul’pina, L. S., Ikonnikov, N. S., & Shul’pin, G. B. (2021). Vanadium(IV) Complexes with Methyl-Substituted 8-Hydroxyquinolines: Catalytic Potential in the Oxidation of Hydrocarbons and Alcohols with Peroxides and Biological Activity. Molecules, 26(21), 6364. https://doi.org/10.3390/molecules26216364