Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil—Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content
Abstract
:1. Introduction
2. Results
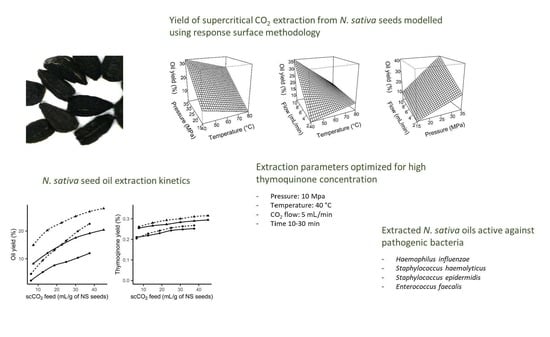
2.1. Modelling of Oil Extraction from Nigella sativa Seeds
2.2. Kinetics of Extraction
2.3. Composition of Nigella sativa Seed Oil Fatty Acids
2.4. Antibacterial Activity of Nigella sativa Seed Extracts
3. Discussion
4. Materials and Methods
4.1. Optimization of Supercritical Carbon Dioxide Extraction from Nigella sativa Seeds
4.1.1. Plant Material and Extraction Equipment
4.1.2. Modelling of Oil Extraction from the Nigella sativa Seeds
4.1.3. Kinetics of Thymoquinone and Oil Extraction
4.2. Oil Composition Analyses
4.2.1. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
4.2.2. Gas Chromatography Fatty Acid Analysis
4.3. Antibacterial Activity of Nigella sativa Seed Oil
4.3.1. Bacterial Strains
4.3.2. Preparation of Nigella sativa Seed Oils for Evaluation of Antibacterial Activity
4.3.3. Minimum Inhibitory Concentration Determinations
4.3.4. Minimum Bactericidal Concentration Determinations
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.T. Analysis of glycolipids from black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) oilseeds. Food Chem. 2003, 80, 197–204. [Google Scholar] [CrossRef]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phyther. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Khan, M.A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 1999, 7, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Khan, A.M.; Karim, S.; Kamal, M.A.; Damanhouri, G.A.; Mirza, Z. Panacea seed “Nigella”: A review focusing on regenerative effects for gastric ailments. Saudi J. Biol. Sci. 2016, 23, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Khalife, K.H.; Lupidi, G. Reduction of hypervalent states of myoglobin and hemoglobin to their ferrous forms by thymoquinone: The role of GSH, NADH and NADPH. Biochim. Biophys. Acta. 2008, 1780, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M.; Al-Bekairi, M.A. Effects of Thymoquinone on Antioxidant Enzyme Activities, Lipid Peroxidation and Dt-Diaphorase in Different Tissues of Mice; A Possible Mechanism of Action. Cell Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Abukhader, M. Thymoquinone in the clinical treatment of cancer: Fact or fiction? Pharmacogn. Rev. 2013, 7, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, M.N.; Gilani, A.H.; Janssen, L.J. Intestinal, airway, and cardiovascular relaxant activities of thymoquinone. Evid.-Based Complement. Altern. Med. 2012, 2012, 305319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.U.; Ashfaq, M.K.; Zuberi, H.S.; Mahmood, M.S.; Gilani, A.H. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother. Res. 2003, 17, 183–186. [Google Scholar] [CrossRef]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Gawron, G.; Krzyczkowski, W.; Lemke, K.; Ołdak, A.; Kadziński, L.; Banecki, B. Nigella sativa seed extract applicability in preparations against methicillin-resistant Staphylococcus aureus and effects on human dermal fibroblasts viability. J. Ethnopharmacol. 2019, 244, 112135. [Google Scholar] [CrossRef]
- Jrah Harzallah, H.; Kouidhi, B.; Flamini, G.; Bakhrouf, A.; Mahjoub, T. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011, 129, 1469–1474. [Google Scholar] [CrossRef]
- Kokoska, L.; Havlik, J.; Valterova, I.; Sovova, H.; Sajfrtova, M.; Jankovska, I. Comparison of Chemical Composition and Antibacterial Activity of Nigella sativa Seed Essential Oils Obtained by Different Extraction Methods. J. Food Prot. 2008, 71, 2475–2480. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Bowyer, M.C.; Van Vuong, Q.; Altena, I.A.; Van Scarlett, C.J. Phytochemicals and antioxidant capacity of Xao tam phan (Paramignya trimera) root as affected by various solvents and extraction methods. Ind. Crops Prod. 2015, 67, 192–200. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Extraction of rotenoids from Derris elliptica using supercritical CO2. J. Chem. Technol. Biotechnol. 2018, 93, 3656–3660. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Hussin, A.S.M. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid.-Based Complement. Altern. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [Green Version]
- Salea, R.; Widjojokusumo, E.; Hartanti, A.W.; Veriansyah, B.; Tjandrawinata, R.R. Supercritical fluid carbon dioxide extraction of Nigella sativa (black cumin) seeds using taguchi method and full factorial design. Biochem. Compd. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Solati, Z.; Baharin, B.S.; Bagheri, H. Supercritical carbon dioxide (SC-CO2) extraction of Nigella sativa L. oil using full factorial design. Ind. Crops Prod. 2012, 36, 519–523. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Porcedda, S.; Falconieri, D.; Dessì, M.A.; Ozcelik, B.; Koca, U. Chemical composition and in vitro bioactivity of the volatile and fixed oils of Nigella sativa L. extracted by supercritical carbon dioxide. Ind. Crops Prod. 2013, 46, 317–323. [Google Scholar] [CrossRef]
- Venkatachallam, S.K.T.; Pattekhan, H.; Divakar, S.; Kadimi, U.S. Chemical composition of Nigella sativa L. seed extracts obtained by supercritical carbon dioxide. J. Food Sci. Technol. 2010, 47, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Sovova, H.; Sajfrtova, M.; Topiar, M. Supercritical CO2 extraction of volatile thymoquinone from Monarda didyma and M. fistulosa herbs. J. Supercrit. Fluids 2015, 105, 29–34. [Google Scholar] [CrossRef]
- Goel, S.; Mishra, P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018, 102, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.; Paul-Satyaseela, M.; Gnanamani, A. In vitro profiling of antimethicillin-resistant Staphylococcus aureus activity of thymoquinone against selected type and clinical strains. Lett. Appl. Microbiol. 2016, 62, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouidhi, B.; Zmantar, T.; Jrah, H.; Souiden, Y.; Chaieb, K.; Mahdouani, K.; Bakhrouf, A. Antibacterial and resistance-modifying activities of thymoquinone against oral pathogens. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| No | X1, Pressure (MPa) | X2, Temperature (°C) | X3, Time (min) | X4, scCO2 Flow (mL/min) | NS Oil Yield (%) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | 20 (−1) | 50 (−1) | 6 (−1) | 4 (−1) | 7.03 | 7.51 |
| 2 | 20 (−1) | 50 (−1) | 6 (−1) | 8 (1) | 16.47 | 17.07 |
| 3 | 20 (−1) | 50 (−1) | 10 (1) | 4 (−1) | 9.58 | 10.23 |
| 4 | 20 (−1) | 50 (−1) | 10 (1) | 8 (1) | 18.63 | 19.79 |
| 5 | 20 (−1) | 70 (1) | 6 (−1) | 4 (−1) | 6.79 | 6.77 |
| 6 | 20 (−1) | 70 (1) | 6 (−1) | 8 (1) | 7.62 | 9.53 |
| 7 | 20 (−1) | 70 (1) | 10 (1) | 4 (−1) | 7.65 | 9.49 |
| 8 | 20 (−1) | 70 (1) | 10 (1) | 8 (1) | 8.71 | 12.25 |
| 9 | 30 (1) | 50 (−1) | 6 (−1) | 4 (−1) | 15.95 | 16.70 |
| 10 | 30 (1) | 50 (−1) | 6 (−1) | 8 (1) | 31.15 | 30.66 |
| 11 | 30 (1) | 50 (−1) | 10 (1) | 4 (−1) | 20.91 | 19.42 |
| 12 | 30 (1) | 50 (−1) | 10 (1) | 8 (1) | 30.05 | 33.38 |
| 13 | 30 (1) | 70 (1) | 6 (−1) | 4 (−1) | 17.13 | 15.96 |
| 14 | 30 (1) | 70 (1) | 6 (−1) | 8 (1) | 23.59 | 23.12 |
| 15 | 30 (1) | 70 (1) | 10 (1) | 4 (−1) | 17.65 | 18.68 |
| 16 | 30 (1) | 70 (1) | 10 (1) | 8 (1) | 24.89 | 25.84 |
| 17 | 15 (-α) | 60 (0) | 8 (0) | 6 (0) | 10.62 | 5.89 |
| 18 | 35 (α) | 60 (0) | 8 (0) | 6 (0) | 29.69 | 28.67 |
| 19 | 25 (0) | 40 (-α) | 8 (0) | 6 (0) | 22.07 | 21.42 |
| 20 | 25 (0) | 80 (α) | 8 (0) | 6 (0) | 15.05 | 13.14 |
| 21 | 25 (0) | 60 (0) | 4 (-α) | 6 (0) | 11.22 | 14.56 |
| 22 | 25 (0) | 60 (0) | 12 (α) | 6 (0) | 21.35 | 19.99 |
| 23 | 25 (0) | 60 (0) | 8 (0) | 2 (-α) | 6.13 | 8.92 |
| 24 | 25 (0) | 60 (0) | 8 (0) | 10 (α) | 27.08 | 25.64 |
| 25 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 17.71 | 17.28 |
| 26 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 18.54 | 17.28 |
| 27 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 18.68 | 17.28 |
| 28 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 17.55 | 17.28 |
| 29 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 21.15 | 17.28 |
| 30 | 25 (0) | 60 (0) | 8 (0) | 6 (0) | 18.44 | 17.28 |
| Source | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Model | 1415 | 6 | 226 | 49.2 | <0.0001 |
| A—scCO2 pressure | 782 | 1 | 782 | 163 | <0.0001 |
| B—scCO2 temperature | 103 | 1 | 103 | 21.6 | <0.0001 |
| C—Extraction time | 44.3 | 1 | 44.3 | 9.25 | 0.0058 |
| D—scCO2 flow | 419 | 1 | 419 | 87.6 | <0.0001 |
| AD | 19.5 | 1 | 19.5 | 4.07 | 0.0555 |
| BD | 46.4 | 1 | 46.4 | 9.68 | 0.0049 |
| Residual | 110 | 23 | 4.79 | ||
| Lack of Fit | 102 | 18 | 5.65 | 3.37 | 0.0918 |
| Pure Error | 8.40 | 5 | 1.68 | ||
| Cor Total | 1525 | 29 |
| Pressure (MPa) | scCO2 Flow (mL/min) | Logarithmic Equation | Power Equation | R2 |
|---|---|---|---|---|
| 10 | 5 | lnY = −0.893·lnX + 2.974 | Y = 19.0·X−0.893 | 0.9979 |
| 10 | 10 | lnY = −0.843·lnX + 2.896 | Y = 18.1·X−0.843 | 0.9987 |
| 15 | 5 | lnY = −0.829·lnX + 2.754 | Y = 15.7·X−0.829 | 0.9997 |
| 15 | 10 | lnY = −0.704·lnX + 2.454 | Y = 11.6·X−0.704 | 0.9972 |
| Pressure (MPa) | Temperature (°C) | Time (min) | scCO2 Flow (mL/min) | C14:0 (%) | C16:0 (%) | C18:2 (%) | C18:1 (%) | C18:0 (%) | C20:2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 50 | 6 | 4 | 0.2 | 11.4 | 60.9 | 22.6 | 2.4 | 2.5 |
| 20 | 50 | 6 | 8 | 0.2 | 12.5 | 60.8 | 21.8 | 2.4 | 2.3 |
| 20 | 50 | 10 | 4 | 0.2 | 12.6 | 61.3 | 21.5 | 2.3 | 2.0 |
| 20 | 50 | 10 | 8 | 0.2 | 12.2 | 60.6 | 22.2 | 2.4 | 2.4 |
| 20 | 70 | 6 | 4 | 0.3 | 13.8 | 57.4 | 23.8 | 2.7 | 2.1 |
| 20 | 70 | 6 | 8 | 0.3 | 13.3 | 58.0 | 24.0 | 2.4 | 2.0 |
| 20 | 70 | 10 | 4 | 0.2 | 12.4 | 57.5 | 25.2 | 2.5 | 2.2 |
| 20 | 70 | 10 | 8 | 0.2 | 13.2 | 60.1 | 21.8 | 2.4 | 2.2 |
| 30 | 50 | 6 | 4 | 0.2 | 11.4 | 61.3 | 22.3 | 2.3 | 2.5 |
| 30 | 50 | 6 | 8 | 0.2 | 11.4 | 61.1 | 22.5 | 2.3 | 2.5 |
| 30 | 50 | 10 | 4 | 0.2 | 11.9 | 60.7 | 22.3 | 2.5 | 2.5 |
| 30 | 50 | 10 | 8 | 0.2 | 11.9 | 60.6 | 22.3 | 2.5 | 2.5 |
| 30 | 70 | 6 | 4 | 0.2 | 11.5 | 60.1 | 23.4 | 2.4 | 2.4 |
| 30 | 70 | 6 | 8 | 0.2 | 11.8 | 59.8 | 23.2 | 2.5 | 2.5 |
| 30 | 70 | 10 | 4 | 0.2 | 11.6 | 59.7 | 23.7 | 2.4 | 2.3 |
| 30 | 70 | 10 | 8 | 0.2 | 11.7 | 60.1 | 23.1 | 2.5 | 2.4 |
| 15 | 60 | 8 | 6 | 0.2 | 11.7 | 61.2 | 22.5 | 2.2 | 2.2 |
| 35 | 60 | 8 | 6 | 0.2 | 11.9 | 60.6 | 22.3 | 2.4 | 2.5 |
| 25 | 40 | 8 | 6 | 0.2 | 10.6 | 62.3 | 22.3 | 2.1 | 2.5 |
| 25 | 80 | 8 | 6 | 0.2 | 12.0 | 60.8 | 22.3 | 2.3 | 2.3 |
| 25 | 60 | 4 | 6 | 0.3 | 11.4 | 60.6 | 22.8 | 2.5 | 2.5 |
| 25 | 60 | 12 | 6 | 0.2 | 12.0 | 60.9 | 22.2 | 2.3 | 2.4 |
| 25 | 60 | 8 | 2 | 0.2 | 11.4 | 61.1 | 22.5 | 2.3 | 2.4 |
| 25 | 60 | 8 | 10 | 0.2 | 12.5 | 58.7 | 23.3 | 2.7 | 2.6 |
| 25 | 60 | 8 | 6 | 0.2 | 11.9 | 60.7 | 22.4 | 2.4 | 2.5 |
| Strain | NSE1 (mg/mL) | NSE2 (mg/mL) | TQ (µg/mL) | AMC/DCBA (µg/mL) | CHQ (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| H. influenzae ATCC 43065 | 0.04 | 0.08 | 0.20 | 0.40 | 4 | 8 | 128 | 128 | 4 | 16 |
| S. haemolyticus ATCC 29970 | 0.08 | 0.08 | 0.40 | 0.40 | 8 | 8 | 128 | 128 | 4 | 32 |
| S. epidermidis ATCC 14990 | 0.08 | 0.32 | 0.4 | 1.6 | 8 | 32 | 32 | 32 | 16 | 64 |
| E. faecalis ATCC 19433 | 0.16 | 0.32 | 0.80 | 1.60 | 16 | 32 | 64 | 128 | 8 | 16 |
| E. coli ATCC 25922 | 1.28 | 1.28 | 6.40 | 6.40 | 128 | 128 | 256 | 512 | 16 | 64 |
| S. sonnei ATCC 9290 | 2.56 | >5.12 | 12.80 | >25.6 | 256 | >512 | 128 | 256 | 16 | 64 |
| S. odorifera ATCC 33077 | 5.12 | >5.12 | 25.60 | >25.6 | 512 | >512 | 128 | 512 | 64 | 64 |
| S. typhimurium ATCC 13311 | 5.12 | >5.12 | 25.60 | >25.6 | 512 | >512 | 64 | 128 | 32 | 64 |
| Independent Variables | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| X1—scCO2 pressure (MPa) | 15 | 20 | 25 | 30 | 35 |
| X2—scCO2 temperature (°C) | 40 | 50 | 60 | 70 | 80 |
| X3—Extraction time (min) | 4 | 6 | 8 | 10 | 12 |
| X4—scCO2 flow (mL/min) | 2 | 4 | 6 | 8 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawron, G.; Krzyczkowski, W.; Łyżeń, R.; Kadziński, L.; Banecki, B. Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil—Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content. Molecules 2021, 26, 6419. https://doi.org/10.3390/molecules26216419
Gawron G, Krzyczkowski W, Łyżeń R, Kadziński L, Banecki B. Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil—Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content. Molecules. 2021; 26(21):6419. https://doi.org/10.3390/molecules26216419
Chicago/Turabian StyleGawron, Grzegorz, Wojciech Krzyczkowski, Robert Łyżeń, Leszek Kadziński, and Bogdan Banecki. 2021. "Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil—Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content" Molecules 26, no. 21: 6419. https://doi.org/10.3390/molecules26216419
APA StyleGawron, G., Krzyczkowski, W., Łyżeń, R., Kadziński, L., & Banecki, B. (2021). Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil—Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content. Molecules, 26(21), 6419. https://doi.org/10.3390/molecules26216419