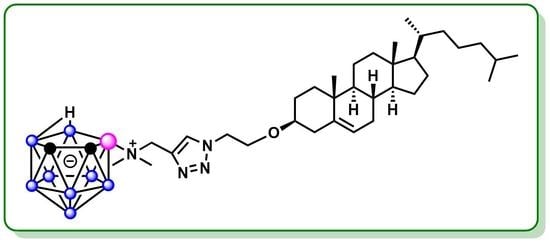
Synthesis of Zwitter-Ionic Conjugate of Nido-Carborane with Cholesterol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Nido-Carboranyl Cholesterol Bearing a 1,2,3-Triazole Fragment
2.2. Single-Crystal X-ray Diffraction Studies
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of the Compounds 4 and 6
3.3. Synthesis of 9-Me2N(CH2)2Me2N-Nido-7,8-C2B9H11 (4)
3.4. Synthesis of 9-3β-Chol-O(CH2)2N3-CH-C-(CH2)Me2N-Nido-7,8-C2B9H11 (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hosmane, N.S.; Eagling, R. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine; World Scientific: London, UK, 2019; ISBN 978-981-4338-67-7. [Google Scholar]
- Cui, P.F.; Liu, X.R.; Guo, S.T.; Lin, Y.J.; Jin, G.X. Steric effects-directed B–H bond activation of para-carboranes. J. Am. Chem. Soc. 2021, 143, 5099–5105. [Google Scholar] [CrossRef]
- Cui, P.F.; Lin, Y.J.; Li, Z.H.; Jin, G.X. Dihydrogen bond interaction induced separation of hexane isomers by self-assembled carborane metallacycles. J. Am. Chem. Soc. 2020, 142, 8532–8538. [Google Scholar] [CrossRef] [PubMed]
- Hosmane, N.S.; Maguire, J.A. Comprehensive Organometallic Chemistry III; Michael, D., Mingos, P., Crabtree, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; p. 264. ISBN 978-0-0804-4601-1. [Google Scholar]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π-Aromaticity and three-dimensional aromaticity: Two sides of the same coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Teixidor, F.; Kivekäs, R.; Sillanpää, R.; Viñas, C. Influence of the solvent and R groups on the structure of (carboranyl)R2PI2 compounds in solution. Crystal structure of the first iodophosphonium salt incorporating the anion [7,8-nido-C2B9H10]−. Dalton Trans. 2008, 11, 1471–1480. [Google Scholar] [CrossRef]
- Kolel-Veetil, M.K.; Keller, T.M. Formation of elastomeric network polymers from ambient heterogeneous hydrosilations of carboranylenesiloxane and branched siloxane monomers. Polym. Sci. Part A 2006, 44, 147–155. [Google Scholar] [CrossRef]
- Řezačova, P.; Cigler, P.; Matejiček, P.; Lipšik, M.; Pokorna, J.; Grüner, B.; Konvalinka, J. Medicinal Application of Carboranes: Inhibition of HIV Protease. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 41. ISBN 9781439826621. [Google Scholar]
- Hosmane, N.S.; Eagling, R. Handbook of Boron Chemistry in Organometallics Catalysis, Materials and Medicine; World Science Publishers: Hackensack, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Kaszynski, P. closo-Boranes as Structural Elements for Liquid Crystals. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 319. ISBN 9781439826621. [Google Scholar]
- Sauerwein, W.A.G. Neutron Capture Therapy: Principles and Applications; Sauerwein, W.A.G., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin, Germany, 2012; p. 554. ISBN 978-3-642-31333-2. [Google Scholar]
- Bregadze, V.I.; Sivaev, I.B. Polyhedral Boron Compounds for BNCT. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 181. ISBN 9781439826621. [Google Scholar]
- Sibrian-Vazquez, M.; Vicente, M.G.H. Boron Tumor Delivery for BNCT: Recent Developments and Perspectives. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 209. ISBN 9781439826621. [Google Scholar]
- Lesnikowski, Z.J. Boron units as pharmacophores-New applications and opportunities of boron cluster chemistry. Collect. Czechoslov. Chem. Commun. 2007, 72, 1646–1658. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 11, 1433–1450. [Google Scholar] [CrossRef]
- Tsygankova, A.R.; Kanygin, V.V.; Kasatova, A.I.; Zavyalov, E.L.; Guselnikova, T.Y.; Kichigin, A.I.; Mukhamadiyarov, R.A. Determination of boron by inductively coupled plasma atomic emission spectroscopy. Biodistribution of 10B in tumor-bearing mice. Russ. Chem. Bull. Int. Ed. 2020, 69, 601–607. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Boron neutron capture therapy. Chemical aspect. Ross. Khim. Zh. 2004, 48, 109. [Google Scholar]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Derivatives of the closo-dodecaborate anion and their application in medicine. Russ. Chem. Bull. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Cabrera-Gonzalez, J.; Xochitiotzi-Florez, E.; Vinas, C.; Teixidor, F.; Garcia, H.; Garcí a-Ortega, H.; Farfan, N.; Santillan, R.; Parella, T.; Nunez, R. High-boron-content porphyrin-cored aryl ether dendrimers: Controlled synthesis, characterization, and photophysical properties. Inorg. Chem. 2015, 54, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.; Haj Ahmad, R.; Ibegbu, D.; Smith, J.; Elkordy, A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, N.H.; Chupin, V.V.; Prokhorov, D.I.; Zhdanova, K.A.; Shvets, V.I. Liposomal delivery systems of biologically active compounds in the treatment of certain diseases. J. Fine Chem. Technol. 2014, 9, 26–41. (In Russian) [Google Scholar]
- Heber, E.M.; Hawthorne, M.F.; Kueffer, P.J.; Garabalino, M.A.; Thorp, S.I.; Pozzi, E.C.; Hughes, A.M.; Maitz, C.A.; Jalisatgi, S.S.; Nigg, D.W.; et al. Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc. Natl. Acad. Sci. USA 2014, 111, 16077–16081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitz, C.A.; Khan, A.A.; Kueffer, P.J.; Brockman, J.D.; Dixson, J.; Jalisatgi, S.S.; Nigg, D.W.; Everett, T.A.; Hawthorne, M.F. Validation and comparison of the therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes in multiple murine tumor models. Transl. Oncol. 2017, 10, 686–692. [Google Scholar] [CrossRef]
- Bialek-Pietras, M.; Olejniczak, A.B.; Tachikawa, S.; Nakamura, H.; Lesnikowski, Z.J. Towards new boron carriers for boron neutron capture therapy: Metallacarboranes bearing cobalt, iron and chromium and their cholesterol conjugates. Bioorg. Med. Chem. 2013, 21, 1136. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef]
- Dubey, R.D.; Sarkar, A.; Shen, Z.; Bregadze, V.I.; Sivaev, I.B.; Druzina, A.A.; Zhidkova, O.B.; Shmal’ko, A.V.; Kosenko, I.D.; Sreejyothi, P.; et al. Effects of linkers on the development of liposomal formulation of cholesterol conjugated cobalt bis(dicarbollides). J. Pharm. Sci. 2020, 110, 1365–1373. [Google Scholar] [CrossRef]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef]
- Giovenzana, G.B.; Lay, L.; Monti, D.; Palmisano, G.; Panza, L. Synthesis of carboranyl derivatives of alkynyl glycosides as potential BNCT agents. Tetrahedron 1999, 55, 14123–14136. [Google Scholar] [CrossRef]
- Ristori, S.; Salvati, A.; Martini, G.; Spalla, O.; Pietrangeli, D.; Posa, A.; Ricciardi, G. Synthesis and liposome insertion of a new poly(carboranylalkylthio)porphyrazine to improve potentiality in multiple-approach cancer therapy. J. Am. Chem. Soc. 2007, 129, 2728–2729. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Balzi, M.; Bortolussi, S.; Bruschi, P.; Ciani, L.; Clerici, A.M.; Faraoni, P.; Ferrari, C.; Gadan, M.A.; Panza, L.; et al. Carborane derivatives loaded into liposomes as efficient delivery systems for boron neutron capture therapy. J. Med. Chem. 2009, 52, 7829–7835. [Google Scholar] [CrossRef]
- Druzina, A.A.; Kosenko, I.D.; Zhidkova, O.B.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Novel cobalt bis(dicarbollide) based on terminal alkynes and their “click” reactions. Eur. J. Inorg. Chem. 2020, 27, 2658–2665. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Kosenko, I.D.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Synthesis and structure of nido-carboranyl azide and its “click” reactions. Molecules 2021, 26, 530–544. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Andrysiak, A.; Grüner, B.; Lesnikowski, Z.J. “Chemical ligation”: A versatile method for nucleoside modification with boron clusters. Chem. Eur. J. 2008, 14, 10675–10682. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Semioshkin, A.A.; Las´kova, J.N.; Berzina, M.Y.; Lobanova, I.A.; Sivaev, I.B.; Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Ulybina, O.V.; et al. Novel types of boronated chlorine e6 conjugates via «click chemistry». Appl. Organometal. Chem. 2009, 23, 370–374. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Andreichuk, E.P.; Zhidkova, O.B.; Kosenko, I.D.; Semioshkin, A.A.; Sivaev, I.B.; Mandal, S.; Shen, Z.; Bregadze, V.I. “Click” synthesis of cobalt bis(dicarbollide)-cholesterol conjugates. Mendeleev Commun. 2019, 29, 628–630. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Kosenko, I.D. Synthesis of conjugates of closo-dodecaborate dianion with cholesterol using a “click” reaction. Russ. Chem. Bull. Int. Ed. 2020, 69, 1080–1084. [Google Scholar] [CrossRef]
- Druzina, A.A.; Stogniy, M.Y. Synthesis of cholesterol derivatives based on closo- and nido-carboranes. Chem. Bull. Int. Ed. 2021, 70, 527–532. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Zhidkova, O.B.; Prikaznova, E.A.; Sivaev, I.B.; Semioshkin, A.; Godovikov, I.A.; Starikova, Z.A.; Bregadze, V.I. Direct synthesis of nido-carborane derivatives with pendant functional groups by copper-promoted reactions with dimethylalkylamines. J. Organomet. Chem. 2014, 757, 21. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.W.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Anisimov, A.A.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. 1,12-Diiodo-ortho-carborane: A classic textbook example of the dihalogen bond. Crystals 2021, 11, 396. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Masunov, A.E.; Antipin, M.Y. Computational search for nonlinear optical materials: Are polarization functions important in the hyperpolarizability predictions of molecules and aggregates? Mendeleev Commun. 2009, 19, 311–313. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Tafur, S.; Masunov, A.E. Applicability of hybrid density functional theory methods to calculation of molecular hyperpolarizability. J. Chem. Phys. 2008, 129, 044109. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Zhang, S. Synthesis of triazole-linked glycoconjugates by copper(I)-catalyzed regiospecific cycloaddition of alkynes and azides. Synth. Commun. 2009, 39, 830–844. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]



| Torsion Angle or H…H Contact | 9-Me2N(CH2)2Me2N-Nido-7,8-C2B9H11 4 | 9-NC≡CCH2Me2N-Nido-7,8-C2B9H11 | 9-PhCH2Me2N-Nido-7,8-C2B9H11 | |||
|---|---|---|---|---|---|---|
| X-ray | DFT | X-ray | DFT | X-ray | DFT | |
| C8-B9-N1-C1 | 19.4(2) | 28.3 | 50.4(2) | 36.0 | 35.3(2) | 41.9 |
| C8-B9-N1-C2 | 138.4(2) | 148.0 | 172.4(2) | 157.8 | 155.8(2) | 162.2 |
| C8-B9-N1-C3 | −103.6(2) | −94.7 | −69.2(2) | −84.1 | −84.8(2) | −78.8 |
| B9-N1-C3-C4 | 64.0(2) | 63.9 | 177.4(2) | 179.6 | 171.9(2) | −179.7 |
| N1-C3-C4-N2 | 178.3(2) | −170.6 | – | – | – | – |
| C3-C4-N2-C5 | 74.1(2) | 81.5 | – | – | – | – |
| C3-C4-N2-C6 | −163.1(2) | −152.8 | – | – | – | – |
| H4…H1A | 2.33 | 2.29 | 2.26 | 2.24 | 2.33 | 2.27 |
| H4A…H12 | 2.38 | 2.39 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Nekrasova, N.A.; Suponitsky, K.Y.; Timofeev, S.V.; Bregadze, V.I. Synthesis of Zwitter-Ionic Conjugate of Nido-Carborane with Cholesterol. Molecules 2021, 26, 6687. https://doi.org/10.3390/molecules26216687
Druzina AA, Zhidkova OB, Dudarova NV, Nekrasova NA, Suponitsky KY, Timofeev SV, Bregadze VI. Synthesis of Zwitter-Ionic Conjugate of Nido-Carborane with Cholesterol. Molecules. 2021; 26(21):6687. https://doi.org/10.3390/molecules26216687
Chicago/Turabian StyleDruzina, Anna A., Olga B. Zhidkova, Nadezhda V. Dudarova, Natalia A. Nekrasova, Kyrill Yu. Suponitsky, Sergey V. Timofeev, and Vladimir I. Bregadze. 2021. "Synthesis of Zwitter-Ionic Conjugate of Nido-Carborane with Cholesterol" Molecules 26, no. 21: 6687. https://doi.org/10.3390/molecules26216687
APA StyleDruzina, A. A., Zhidkova, O. B., Dudarova, N. V., Nekrasova, N. A., Suponitsky, K. Y., Timofeev, S. V., & Bregadze, V. I. (2021). Synthesis of Zwitter-Ionic Conjugate of Nido-Carborane with Cholesterol. Molecules, 26(21), 6687. https://doi.org/10.3390/molecules26216687