Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Optical Properties
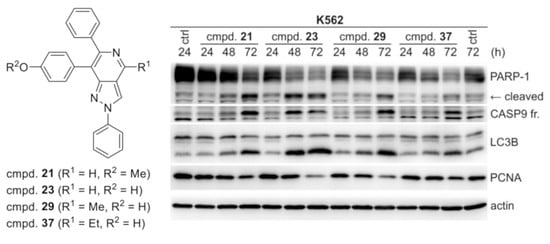
2.3. Biology
3. Materials and Methods
3.1. General
3.2. Chemistry
3.2.1. Procedure for the Synthesis of [1-Phenyl-3-(phenylethynyl)-1H-pyrazol-4-yl]methanol 3
3.2.2. General Procedure (A) for the Synthesis of Alcohols 4–7
1-[1-Phenyl-3-(phenylethynyl)-1H-pyrazol-4-yl]ethanol-1-ol 4
1-[1-Phenyl-3-(phenylethynyl)-1H-pyrazol-4-yl]propan-1-ol 5
2-Methyl-1-[1-phenyl-3-(phenylethynyl)-1H-pyrazol-4-yl]propan-1-ol 6
Phenyl[1-phenyl-3-(phenylethynyl)-1H-pyrazol-4-yl]methanol 7
3.2.3. General Procedure (B) for the Synthesis of Azide–Alkynes 8–12
4-(Azidomethyl)-1-phenyl-3-(phenylethynyl)-1H-pyrazole 8
4-(1-Azidoethyl)-1-phenyl-3-(phenylethynyl)-1H-pyrazole 9
4-(1-Azidopropyl)-1-phenyl-3-(phenylethynyl)-1H-pyrazole 10
4-(Azido-2-methylpropyl)-1-phenyl-3-(phenylethynyl)-1H-pyrazole 11
4-[Azido(phenyl)methyl]-1-phenyl-3-(phenylethynyl)-1H-pyrazole 12
3.2.4. General Procedure (C) for the Synthesis of 7-Iodo-2H-pyrazolo[4,3-c]pyridines 13–17
7-Iodo-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 13
7-Iodo-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 14
4-Ethyl-7-iodo-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 15
7-Iodo-4-isopropyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 16
7-Iodo-2,4,6-triphenyl-2H-pyrazolo[4,3-c]pyridine 17
3.2.5. General Procedure (D) for the Synthesis of 7-Substituted Pyrazolo[4,3-c]pyridine derivatives 18–39 by Suzuki–Miyaura Cross-Coupling with Boronic acids
2,6,7-Triphenyl-2H-pyrazolo[4,3-c]pyridine 18
7-(2-Methoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 19
7-(3-Methoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 20
7-(4-Methoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 21
7-(3,4-Dimethoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 22
4-(2,6-Diphenyl-2H-pyrazolo[4,3-c]pyridin-7-yl)phenol 23
4-Methyl-2,6,7-triphenyl-2H-pyrazolo[4,3-c]pyridine 24
7-(2-Methoxyphenyl)-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 25
7-(3-Methoxyphenyl)-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 26
7-(4-Methoxyphenyl)-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 27
7-(3,4-Dimethoxyphenyl)-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 28
4-(4-Methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridin-7-yl)phenol 29
4-Ethyl-2,6,7-triphenyl-2H-pyrazolo[4,3-c]pyridine 30
4-Ethyl-7-(4-methoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 31
7-(2,4-Dimethoxyphenyl)-4-ethyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 32
4-Ethyl-2,6-diphenyl-7-(p-tolyl)-2H-pyrazolo[4,3-c]pyridine 33
4-Ethyl-2,6-diphenyl-7-[4-(trifluoromethyl)phenyl]-2H-pyrazolo[4,3-c]pyridine 34
4-Ethyl-2,6-diphenyl-7-[4-(trifluoromethoxy)phenyl]-2H-pyrazolo[4,3-c]pyridine 35
7-(4-Chlorophenyl)-4-ethyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 36
4-(4-Ethyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridin-7-yl)phenol 37
4-Isopropyl-7-(4-methoxyphenyl)-4-methyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 38
7-(4-Methoxyphenyl)-2,4,6-triphenyl-2H-pyrazolo[4,3-c]pyridine 39
3.2.6. General Procedure (E) of 4-(4-Ethyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridin-7-yl)phenol 37 Alkylation
7-(4-Ethoxyphenyl)-4-ethyl-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 40
4-Ethyl-2,6-diphenyl-7-(4-propoxyphenyl)-2H-pyrazolo[4,3-c]pyridine 41
4-Ethyl-7-(4-isopropoxyphenyl)-2,6-diphenyl-2H-pyrazolo[4,3-c]pyridine 42
3.3. Optical Properties
- Stock solutions (4 mM) of the compounds were prepared in DMSO and further diluted in a Britton–Robinson buffer to a final concentration of 2 μM for spectroscopic analyses. Absorption spectra at pH 5, 7, and 9 for all compounds and in the 2–11 pH range with 0.5 step for selected compounds were measured using a Specord 250 Plus spectrophotometer in appropriate Britton–Robinson buffers. The spectra were measured in the 240–450 nm interval with a step of 1 nm, a 1 nm bandpass, and an integration time of 0.5 s. The samples were placed into a quartz cuvette with an optical path of 1 cm. The baseline was measured for the cuvette containing the solvent only.
- The steady-state excitation and emission spectra of 2 μM solutions of all the compounds at pH 5, 7, and 9 and in the 2–11 pH range with a 0.5 step for selected compounds were recorded on a Fluorolog-3 fluorimeter in the quartz cuvette with the 1 cm optical path (both in excitation and emission). Bandpasses in both the excitation and emission monochromator were set to 2 nm, and the spectra were scanned with the 1 nm step and an integration time 0.2 s per data point at 22 °C. Emission spectra were recorded in a 370–700 nm range with excitation at 360 nm.
- The quantum yield was estimated via integration of the fluorescence intensity over a range of 370–700 nm, and a 2.5 μM quinine sulphate solution in 0.05 M H2SO4 was used as a standard (Φf = 60%) [76].
3.4. Biology
3.4.1. Cell Cultures
3.4.2. Antiproliferative Activity Assay
3.4.3. Immunoblotting
3.4.4. Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Shamsuzzaman Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Kucukguzel, S.G.; Senkardes, S. Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 2015, 97, 786–815. [Google Scholar] [CrossRef]
- Varvuolytė, G.; Malina, L.; Bieliauskas, A.; Hošíková, B.; Simerská, H.; Kolářová, H.; Kleizienė, N.; Kryštof, V.; Šačkus, A.; Žukauskaitė, A. Synthesis and photodynamic properties of pyrazole-indole hybrids in the human skin melanoma cell line G361. Dyes Pigment. 2020, 183, 108666. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N.; Dhanalakshmi, C.; Manivasagam, T.; Essa, M.M. CNB-001, a novel pyrazole derivative mitigates motor impairments associated with neurodegeneration via suppression of neuroinflammatory and apoptotic response in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2014, 220, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Milišiūnaitė, V.; Kadlecová, A.; Žukauskaitė, A.; Doležal, K.; Strnad, M.; Voller, J.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. Synthesis and anthelmintic activity of benzopyrano[2,3-c]pyrazol-4(2H)-one derivatives. Mol. Divers. 2020, 24, 1025–1042. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, P.; Pickard, M.R.; Parekh, H.H.; Patel, S.P.; Pathak, R.B. Synthesis and biological evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Org. Biomol. Chem. 2013, 11, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Masih, A.; Agnihotri, A.K.; Srivastava, J.K.; Pandey, N.; Bhat, H.R.; Singh, U.P. Discovery of novel pyrazole derivatives as a potent anti-inflammatory agent in RAW264.7 cells via inhibition of NF-ĸB for possible benefit against SARS-CoV-2. J. Biochem. Mol. Toxicol. 2020, 35, e22656. [Google Scholar] [CrossRef]
- Gogoi, P.; Shakya, A.; Ghosh, S.K.; Gogoi, N.; Gahtori, P.; Singh, N.; Bhattacharyya, D.R.; Singh, U.P.; Bhat, H.R. In silico study, synthesis, and evaluation of the antimalarial activity of hybrid dimethoxy pyrazole 1,3,5-triazine derivatives. J. Biochem. Mol. Toxicol. 2020, 35, e22682. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.F.; El-Badawi, M.A.; Ibrahim, S.A.; El-Borai, M.A. Synthesis of some novel heterocyclic dyes derived from pyrazole derivatives. Arab. J. Chem. 2011, 4, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Karabacak, Ç.; Tilki, T.; Tuncer, B.Ö.; Cengiz, M. Antimicrobial pyrazole dyes: Synthesis, characterization, and absorption characteristics. Res. Chem. Intermed. 2015, 41, 1985–1999. [Google Scholar] [CrossRef]
- Götzinger, A.C.; Theßeling, F.A.; Hoppe, C.; Müller, T.J.J. One-Pot Coupling-Coupling-Cyclocondensation Synthesis of Fluorescent Pyrazoles. J. Org. Chem. 2016, 81, 10328–10338. [Google Scholar] [CrossRef]
- Milišiūnaitė, V.; Arbačiauskienė, E.; Bieliauskas, A.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Synthesis of pyrazolo[4′,3′:3,4]pyrido[1,2-a]benzimidazoles and related new ring systems by tandem cyclisation of vic-alkynylpyrazole-4-carbaldehydes with (het)aryl-1,2-diamines and investigation of their optical properties. Tetrahedron 2015, 71, 3385–3395. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- Nayak, N.; Prasad, K.S.; Pillai, R.R.; Armaković, S.; Armaković, S.J. Remarkable colorimetric sensing behavior of pyrazole-based chemosensor towards Cu(II) ion detection: Synthesis, characterization and theoretical investigations. RSC Adv. 2018, 8, 18023–18029. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.K.; Suresh, M.; Suresh, E.; Mishra, S.K.; Mishra, S.; Das, A. A chemosensor for heavy-transition metal ions in mixed aqueous-organic media. Sens. Actuators B Chem. 2010, 145, 32–38. [Google Scholar] [CrossRef]
- Swami, S.; Agarwala, A.; Behera, D.; Shrivastava, R. Diaminomaleonitrile based chromo-fluorescent receptor molecule for selective sensing of Mn(II) and Zn(II) ions. Sens. Actuators B Chem. 2018, 260, 1012–1017. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. Synthesis, Spectroscopy Studies, and Theoretical Calculations of New Fluorescent Probes Based on Pyrazole Containing Porphyrins for Zn(II), Cd(II), and Hg(II) Optical Detection. Inorg. Chem. 2014, 53, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Garzón, L.-M.; Portilla, J. Synthesis of Novel D-π-A Dyes for Colorimetric Cyanide Sensing Based on Hemicyanine-Functionalized N-(2-Pyridyl)pyrazoles. Eur. J. Org. Chem. 2019, 2019, 7079–7088. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Portilla, J. Synthesis of Dicyanovinyl-Substituted 1-(2-Pyridyl)pyrazoles: Design of a Fluorescent Chemosensor for Selective Recognition of Cyanide. J. Org. Chem. 2017, 82, 13376–13385. [Google Scholar] [CrossRef]
- Lee, H.; Berezin, M.Y.; Tang, R.; Zhegalova, N.; Achilefu, S. Pyrazole-substituted near-infrared cyanine dyes exhibit pH-dependent fluorescence lifetime properties. Photochem. Photobiol. 2013, 89, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Duan, H.; Xing, D.; Yang, G. Novel Turn-on Fluorescence Probes for Al3+ Based on Conjugated Pyrazole Schiff Base. J. Fluoresc. 2017, 27, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Naskar, B.; Das, K.; Mondal, R.R.; Maiti, D.K.; Requena, A.; Cerón-Carrasco, J.P.; Prodhan, C.; Chaudhuri, K.; Goswami, S. A new fluorescence turn-on chemosensor for nanomolar detection of Al 3+ constructed from a pyridine–pyrazole system. New J. Chem. 2018, 42, 2933–2941. [Google Scholar] [CrossRef]
- Islam, A.S.M.; Bhowmick, R.; Mohammad, H.; Katarkar, A.; Chaudhuri, K.; Ali, M. A novel 8-hydroxyquinoline-pyrazole based highly sensitive and selective Al(III) sensor in a purely aqueous medium with intracellular application: Experimental and computational studies. New J. Chem. 2016, 40, 4710–4719. [Google Scholar] [CrossRef]
- Ciupa, A.; Mahon, M.F.; De Bank, P.A.; Caggiano, L. Simple pyrazoline and pyrazole “turn on” fluorescent sensors selective for Cd2+ and Zn2+ in MeCN. Org. Biomol. Chem. 2012, 10, 8753. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yee, K.K.; Lo, K.K.W.; Zhang, K.Y.; To, W.P.; Che, C.M.; Xu, Z. Selective Ag(I) binding, H2S sensing, and white-light emission from an easy-to-make porous conjugated polymer. J. Am. Chem. Soc. 2014, 136, 2818–2824. [Google Scholar] [CrossRef]
- Dhara, A.; Guchhait, N.; Mukherjee, I.; Mukherjee, A.; Chandra Bhattacharya, S. A novel pyrazole based single molecular probe for multi-analyte (Zn2+ and Mg2+) detection in human gastric adenocarcinoma cells. RSC Adv. 2016, 6, 105930–105939. [Google Scholar] [CrossRef]
- Paitandi, R.P.; Sharma, V.; Singh, V.D.; Dwivedi, B.K.; Mobin, S.M.; Pandey, D.S. Pyrazole appended quinoline-BODIPY based arene ruthenium complexes: Their anticancer activity and potential applications in cellular imaging. Dalton Trans. 2018, 47, 17500–17514. [Google Scholar] [CrossRef]
- Paitandi, R.P.; Mukhopadhyay, S.; Singh, R.S.; Sharma, V.; Mobin, S.M.; Pandey, D.S. Anticancer Activity of Iridium(III) Complexes Based on a Pyrazole-Appended Quinoline-Based BODIPY. Inorg. Chem. 2017, 56, 12232–12247. [Google Scholar] [CrossRef]
- Faderl, S.; Pal, A.; Bornmann, W.; Albitar, M.; Maxwell, D.; Van, Q.; Peng, Z.; Harris, D.; Liu, Z.; Hazan-Halevy, I.; et al. Kit inhibitor APcK110 induces apoptosis and inhibits proliferation of acute myeloid leukemia cells. Cancer Res. 2009, 69, 3910–3917. [Google Scholar] [CrossRef] [Green Version]
- Faderl, S.; Bueso-Ramos, C.; Liu, Z.; Pal, A.; Bornmann, W.; Ciurea, D.V.; Harris, D.; Hazan-Halevy, I.; Kantarjian, H.M.; Estrov, Z. Kit inhibitor APcK110 extends survival in an AML xenograft mouse model. Investig. New Drugs 2011, 29, 1094–1097. [Google Scholar] [CrossRef] [Green Version]
- Faderl, S.; Bornmann, W.; Maxwell, D.; Pal, A.; Peng, Z.-H.; Shavrin, A.; Harris, D.; Van, Q.; Zhiming, L.; Verstovsek, S.; et al. APCK110, a Novel and Potent Inhibitor of c-Kit, Blocks Phosphorylation of AKT and STAT3, Induces Apoptosis, and Inhibits Proliferation of Acute Myeloid Leukemia (AML) Cells. Blood 2006, 108, 153. [Google Scholar] [CrossRef]
- Nam, Y.; Hwang, D.; Kim, N.; Seo, H.-S.; Selim, K.B.; Sim, T. Identification of 1H-pyrazolo[3,4-b]pyridine derivatives as potent ALK-L1196M inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 1426–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czodrowski, P.; Mallinger, A.; Wienke, D.; Esdar, C.; Pöschke, O.; Busch, M.; Rohdich, F.; Eccles, S.A.; Ortiz-Ruiz, M.J.; Schneider, R.; et al. Structure-Based Optimization of Potent, Selective, and Orally Bioavailable CDK8 Inhibitors Discovered by High-Throughput Screening. J. Med. Chem. 2016, 59, 9337–9349. [Google Scholar] [CrossRef]
- Yoshida, T.; Oki, H.; Doi, M.; Fukuda, S.; Yuzuriha, T.; Tabata, R.; Ishimoto, K.; Kawahara, K.; Ohkubo, T.; Miyachi, H.; et al. Structural Basis for PPARα Activation by 1H-pyrazolo-[3,4-b]pyridine Derivatives. Sci. Rep. 2020, 10, 7623. [Google Scholar] [CrossRef]
- El-Gohary, N.S.; Gabr, M.T.; Shaaban, M.I. Synthesis, molecular modeling and biological evaluation of new pyrazolo[3,4-b]pyridine analogs as potential antimicrobial, antiquorum-sensing and anticancer agents. Bioorg. Chem. 2019, 89, 102976. [Google Scholar] [CrossRef]
- Park, C.M.; Jadhav, V.B.; Song, J.-H.; Lee, S.; Won, H.Y.; Choi, S.U.; Son, Y.H. 3-Amino-1H-pyrazolopyridine Derivatives as a Maternal Embryonic Leucine Zipper Kinase Inhibitor. Bull. Korean Chem. Soc. 2017, 38, 595–602. [Google Scholar] [CrossRef]
- Howard, S.; Amin, N.; Benowitz, A.B.; Chiarparin, E.; Cui, H.; Deng, X.; Heightman, T.D.; Holmes, D.J.; Hopkins, A.; Huang, J.; et al. Fragment-based discovery of 6-azaindazoles as inhibitors of bacterial DNA ligase. ACS Med. Chem. Lett. 2013, 4, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engers, D.W.; Bollinger, S.R.; Engers, J.L.; Panarese, J.D.; Breiner, M.M.; Gregro, A.; Blobaum, A.L.; Bronson, J.J.; Wu, Y.J.; Macor, J.E.; et al. Discovery and characterization of N-(1,3-dialkyl-1H-indazol-6-yl)-1H-pyrazolo[4,3-b]pyridin-3-amine scaffold as mGlu4 positive allosteric modulators that mitigate CYP1A2 induction liability. Bioorg. Med. Chem. Lett. 2018, 28, 2641–2646. [Google Scholar] [CrossRef]
- Engers, D.W.; Blobaum, A.L.; Gogliotti, R.D.; Cheung, Y.Y.; Salovich, J.M.; Garcia-Barrantes, P.M.; Daniels, J.S.; Morrison, R.; Jones, C.K.; Soars, M.G.; et al. Discovery, Synthesis, and Preclinical Characterization of N-(3-Chloro-4-fluorophenyl)-1H-pyrazolo[4,3-b]pyridin-3-amine (VU0418506), a Novel Positive Allosteric Modulator of the Metabotropic Glutamate Receptor 4 (mGlu4). ACS Chem. Neurosci. 2016, 7, 1192–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannouli, V.; Lougiakis, N.; Kostakis, I.K.; Pouli, N.; Marakos, P.; Skaltsounis, A.-L.; Horne, D.A.; Nam, S.; Gioti, K.; Tenta, R. Design and Synthesis of New Substituted Pyrazolopyridines with Potent Antiproliferative Activity. Med. Chem. 2020, 16, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, M.; Giannouli, V.; Kotsikoris, V.; Papadodima, O.; Kontogianni, G.; Kostakis, I.K.; Lougiakis, N.; Chatziioannou, A.; Kolisis, F.N.; Marakos, P.; et al. Novel pyrazolopyridine derivatives as potential angiogenesis inhibitors: Synthesis, biological evaluation and transcriptome-based mechanistic analysis. Eur. J. Med. Chem. 2016, 121, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Zhou, Y.; Lu, W.Q.; Zhong, Y.; Song, W.L.; Liu, K.D.; Huang, J.; Zhao, Z.J.; Xu, Y.F.; Liu, X.F.; et al. Identification of Inhibitors against p90 ribosomal S6 kinase 2 (RSK2) through structure-based virtual screening with the inhibitor-constrained refined homology model. J. Chem. Inf. Model. 2011, 51, 2939–2947. [Google Scholar] [CrossRef]
- Smyth, L.A.; Matthews, T.P.; Collins, I. Design and evaluation of 3-aminopyrazolopyridinone kinase inhibitors inspired by the natural product indirubin. Bioorg. Med. Chem. 2011, 19, 3569–3578. [Google Scholar] [CrossRef] [PubMed]
- Vilkauskaitė, G.; Schaaf, P.; Šačkus, A.; Krystof, V.; Holzer, W. Synthesis of pyridyl substituted pyrazolo[4,3-c]pyridines as potential inhibitors of protein kinases. Arkivoc 2014, 2014, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Holzer, W.; Vilkauskaitė, G.; Arbačiauskienė, E.; Šačkus, A. Dipyrazolo[1,5-a:4’,3’-c]pyridines—A new heterocyclic system accessed via multicomponent reaction. Beilstein J. Org. Chem. 2012, 8, 2223–2229. [Google Scholar] [CrossRef]
- Palka, B.; Di Capua, A.; Anzini, M.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Synthesis of trifluoromethyl-substituted pyrazolo[4,3-c]pyridines—Sequential versus multicomponent reaction approach. Beilstein J. Org. Chem. 2014, 10, 1759–1764. [Google Scholar] [CrossRef] [Green Version]
- Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Sonogashira-type reactions with 5-chloro-1-phenyl-1H-pyrazole-4-carbaldehydes: A straightforward approach to pyrazolo[4,3-c]pyridines. Eur. J. Org. Chem. 2011, 2011, 5123–5133. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Laukaitytė, V.; Holzer, W.; Šačkus, A. Metal-Free Intramolecular Alkyne-Azide Cycloaddition To Construct the Pyrazolo[4,3-f][1,2,3]triazolo[5,1-c][1,4]oxazepine Ring System. Eur. J. Org. Chem. 2015, 2015, 5663–5670. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Ethyl 3-and 5-triflyloxy-1H-pyrazole-4-carboxylates in the synthesis of condensed pyrazoles by Pd-catalysed cross-coupling reactions. Eur. J. Org. Chem. 2011, 2011, 1880–1890. [Google Scholar] [CrossRef]
- Bieliauskas, A.; Krikštolaitytė, S.; Holzer, W.; Šačkus, A. Ring-closing metathesis as a key step to construct 2,6-dihydropyrano[2,3-c]pyrazole ring system. Arkivoc 2018, 2018, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Milišiūnaitė, V.; Arbačiauskienė, E.; Řezníčková, E.; Jorda, R.; Malínková, V.; Žukauskaitė, A.; Holzer, W.; Šačkus, A.; Kryštof, V. Synthesis and anti-mitotic activity of 2,4- or 2,6-disubstituted- and 2,4,6-trisubstituted-2H-pyrazolo[4,3-c]pyridines. Eur. J. Med. Chem. 2018, 150, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Milišiūnaitė, V.; Plytninkienė, E.; Bakšienė, R.; Bieliauskas, A.; Krikštolaitytė, S.; Račkauskienė, G.; Arbačiauskienė, E.; Šačkus, A. Convenient Synthesis of Pyrazolo[4′,3′:5,6]pyrano[4,3-c][1,2]oxazoles via Intramolecular Nitrile Oxide Cycloaddition. Molecules 2021, 26, 5604. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Martynaitis, V.; Krikštolaitytė, S.; Holzer, W.; Šačkus, A. Synthesis of 3-substituted 1-phenyl-1H-pyrazole-4-carbaldehydes and the corresponding ethanones by Pd-catalysed cross-coupling reactions. Arkivoc 2011, 2011, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Pompeu, T.E.T.; Alves, F.R.S.; Figueiredo, C.D.M.; Antonio, C.B.; Herzfeldt, V.; Moura, B.C.; Rates, S.M.K.; Barreiro, E.J.; Fraga, C.A.M.; Noël, F. Synthesis and pharmacological evaluation of new N-phenylpiperazine derivatives designed as homologues of the antipsychotic lead compound LASSBio-579. Eur. J. Med. Chem. 2013, 66, 122–134. [Google Scholar] [CrossRef]
- Riva, E.; Gagliardi, S.; Martinelli, M.; Passarella, D.; Vigo, D.; Rencurosi, A. Reaction of Grignard reagents with carbonyl compounds under continuous flow conditions. Tetrahedron 2010, 66, 3242–3247. [Google Scholar] [CrossRef]
- Goebel, M.T.; Marvel, C.S. The Oxidation of Grignard Reagents. J. Am. Chem. Soc. 1933, 55, 1693–1696. [Google Scholar] [CrossRef]
- Besset, C.; Chambert, S.; Fenet, B.; Queneau, Y. Direct azidation of unprotected carbohydrates under Mitsunobu conditions using hydrazoic acid. Tetrahedron Lett. 2009, 50, 7043–7047. [Google Scholar] [CrossRef]
- Mitsunobu, O. The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products. Synthesis 1981, 1981, 1–28. [Google Scholar] [CrossRef]
- Bräse, S.; Banert, K. Organic Azides: Syntheses and Applications; John Wiley: New York, NY, USA, 2010; ISBN 9780470519981. [Google Scholar]
- Semina, E.; Žukauskaitė, A.; Šačkus, A.; De Kimpe, N.; Mangelinckx, S. Selective Elaboration of Aminodiols towards Small Ring α- and β-Amino Acid Derivatives that Incorporate an Aziridine, Azetidine, or Epoxide Scaffold. Eur. J. Org. Chem. 2016, 2016, 1720–1731. [Google Scholar] [CrossRef]
- Peterson, T.; Streamland, T.; Awad, A. A Tractable and Efficient One-Pot Synthesis of 5′-Azido-5′-deoxyribonucleosides. Molecules 2014, 19, 2434–2444. [Google Scholar] [CrossRef]
- Kuroda, K.; Hayashi, Y.; Mukaiyama, T. Conversion of tertiary alcohols to tert-alkyl azides by way of quinone-mediated oxidation–reduction condensation using alkyl diphenylphosphinites. Tetrahedron 2007, 63, 6358–6364. [Google Scholar] [CrossRef]
- Fischer, D.; Tomeba, H.; Pahadi, N.K.; Patil, N.T.; Huo, Z.; Yamamoto, Y. Iodine-Mediated Electrophilic Cyclization of 2-Alkynyl-1-methylene Azide Aromatics Leading to Highly Substituted Isoquinolines and Its Application to the Synthesis of Norchelerythrine. J. Am. Chem. Soc. 2008, 130, 15720–15725. [Google Scholar] [CrossRef]
- Žukauskaitė, Ž.; Buinauskaitė, V.; Solovjova, J.; Malinauskaitė, L.; Kveselytė, A.; Bieliauskas, A.; Ragaitė, G.; Šačkus, A. Microwave-assisted synthesis of new fluorescent indoline-based building blocks by ligand free Suzuki-Miyaura cross-coupling reaction in aqueous media. Tetrahedron 2016, 72, 2955–2963. [Google Scholar] [CrossRef]
- Aoi, W.; Marunaka, Y. Importance of pH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. Biomed Res. Int. 2014, 2014, 598986. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Tchaikovskaya, O.N.; Sokolova, I.V.; Kuznetsova, R.T.; Swetlitchnyi, V.A.; Kopylova, T.N.; Mayer, G.V. Fluorescence investigations of phenol phototransformation in aqueous solutions. J. Fluoresc. 2000, 10, 403–408. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Sofotasiou, M.; Papadopoulou, V.; Polonyfi, K.; Iliakis, T.; Viniou, N.A. PARP1-driven apoptosis in chronic lymphocytic leukemia. Biomed Res. Int. 2014, 2014, 106713. [Google Scholar] [CrossRef] [Green Version]
- Brady, S.C.; Allan, L.A.; Clarke, P.R. Regulation of Caspase 9 through Phosphorylation by Protein Kinase C Zeta in Response to Hyperosmotic Stress. Mol. Cell. Biol. 2005, 25, 10543–10555. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.E.; Johansen, T. Following autophagy step by step. BMC Biol. 2011, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.D.; McNally, N.J.; Dische, S.; Saunders, M.I.; Des Rochers, C.; Lewis, A.A.; Bennett, M.H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br. J. Cancer 1988, 58, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]





| Structure | Compound | R1 | R2 | GI50 ± SD, µM * | ||
|---|---|---|---|---|---|---|
| MV4-11 | K562 | MCF-7 | ||||
 | 18 | H | Ph | 7.7 ± 2.6 | >10 | >10 |
| 19 | H | 2-MeO-Ph | 6.5 ± 1.3 | 7.1 ± 2.9 | 6.3 ± 2.2 | |
| 20 | H | 3-MeO-Ph | 5.0 ± 1.8 | >10 | >10 | |
| 21 | H | 4-MeO-Ph | 3.5 ± 1.2 | 4.8 ± 2.5 | 7.3 ± 0.2 | |
| 22 | H | 3,4-di-MeO-Ph | 2.4 ± 1.3 | 6.0 ± 3.8 | 4.2 ± 0.9 | |
| 23 | H | 4-OH-Ph | 1.5 ± 0.7 | 2.4 ± 1.0 | 1.6 ± 0.2 | |
| 24 | Me | Ph | >10 | >10 | >10 | |
| 25 | Me | 2-MeO-Ph | >10 | >10 | >10 | |
| 26 | Me | 3-MeO-Ph | >10 | >10 | >10 | |
| 27 | Me | 4-MeO-Ph | >10 | >10 | 8.4 ± 2.1 | |
| 28 | Me | 3,4-di-MeO-Ph | 7.6 ± 3.0 | >10 | >10 | |
| 29 | Me | 4-OH-Ph | 4.7 ± 2.7 | 3.9 ± 0.3 | 4.1 ± 0.4 | |
| 30 | Et | Ph | >10 | >10 | >10 | |
| 31 | Et | 4-MeO-Ph | >10 | >10 | >10 | |
| 32 | Et | 2,4-di-MeO-Ph | >10 | >10 | >10 | |
| 33 | Et | 4-Me-Ph | >10 | >10 | >10 | |
| 34 | Et | 4-CF3-Ph | >10 | >10 | >10 | |
| 35 | Et | 4-CF3O-Ph | >10 | >10 | >10 | |
| 36 | Et | 4-Cl-Ph | >10 | >10 | >10 | |
| 37 | Et | 4-OH-Ph | 8.0 ± 3.1 | >10 | 3.9 ± 0.4 | |
| 38 | iPr | 4-MeO-Ph | 5.7 ± 1.4 | >10 | >10 | |
| 39 | Ph | 4-MeO-Ph | >10 | >10 | 7.9 ± 3.8 | |
| 40 | Et | 4-EtO-Ph | >10 | >10 | >10 | |
| 41 | Et | 4-PrO-Ph | >10 | >10 | >10 | |
| 42 | Et | 4-iPrO-Ph | >10 | >10 | >10 | |
| Flavopiridol | 0.2 ± 0.03 | 0.8 ± 0.1 | 0.2 ± 0.03 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razmienė, B.; Řezníčková, E.; Dambrauskienė, V.; Ostruszka, R.; Kubala, M.; Žukauskaitė, A.; Kryštof, V.; Šačkus, A.; Arbačiauskienė, E. Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines. Molecules 2021, 26, 6747. https://doi.org/10.3390/molecules26216747
Razmienė B, Řezníčková E, Dambrauskienė V, Ostruszka R, Kubala M, Žukauskaitė A, Kryštof V, Šačkus A, Arbačiauskienė E. Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines. Molecules. 2021; 26(21):6747. https://doi.org/10.3390/molecules26216747
Chicago/Turabian StyleRazmienė, Beatričė, Eva Řezníčková, Vaida Dambrauskienė, Radek Ostruszka, Martin Kubala, Asta Žukauskaitė, Vladimír Kryštof, Algirdas Šačkus, and Eglė Arbačiauskienė. 2021. "Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines" Molecules 26, no. 21: 6747. https://doi.org/10.3390/molecules26216747
APA StyleRazmienė, B., Řezníčková, E., Dambrauskienė, V., Ostruszka, R., Kubala, M., Žukauskaitė, A., Kryštof, V., Šačkus, A., & Arbačiauskienė, E. (2021). Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines. Molecules, 26(21), 6747. https://doi.org/10.3390/molecules26216747