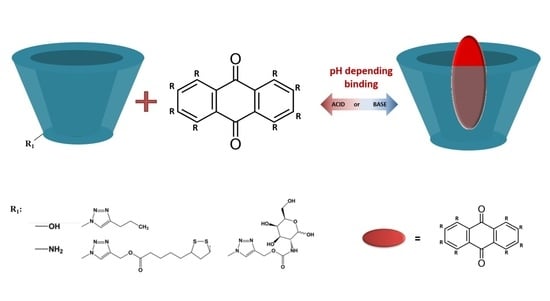
Adjusting the Structure of β-Cyclodextrin to Improve Complexation of Anthraquinone-Derived Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of pKa Values of CD Derivatives
2.2. Tuning β-Cyclodextrin Structure to Improve the Solubilities of AQ2CA and AQ2S Drugs
2.3. Determination of the Association Constants of Daunorubicin–Cyclodextrin Inclusion Complexes at pH 7.4. and 5.5
2.4. Determination of the βCD Complexes Binding Site by Nuclear Magnetic Spectroscopy
3. Material and Methods
3.1. Chemicals and Reagents
3.2. UV-Vis Spectroscopy
3.3. Voltammetry
3.4. Phase Solubility Diagrams
3.5. NMR Measurements
3.6. Evaluation of the Association Constants of Drug–Cyclodextrin Inclusion Complexes by Cyclic and Square-Wave Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Greidanus, J.; Willemse, P.H.; Uges, D.R.; Oremus, E.T.; De Langen, Z.J.; De Vries, E.G. Continuous infusion of low-dose doxorubicin, epirubicin and mitoxantrone in cancer chemotherapy: A review. Pharm. Weekbl. 1988, 10, 237–245. [Google Scholar] [CrossRef]
- Patterson, L.H. Bioreductively activated antitumor N-oxides: The case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab. Rev. 2002, 34, 581–592. [Google Scholar] [CrossRef]
- Andersen, D.O.; Weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Panigrahi, G.K.; Yadav, A.; Mandal, P.; Tripathi, A.; Das, M. Immunomodulatory potential of Rhein, an anthraquinone moiety of Cassia occidentalis seeds. Toxicol. Lett. 2016, 245, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.D.; Panchal, P.V.; Harle, U.N.; Nanda, R.K.; Shaikh, H.M. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents. Int. J. Inflamm. 2014, 2014, 690596. [Google Scholar] [CrossRef] [Green Version]
- Khanal, P.; Patil, B.M.; Chand, J.; Naaz, Y. Anthraquinone Derivatives as an Immune Booster and their Therapeutic Option Against COVID-19. Nat. Prod. Bioprospect. 2020, 10, 325–335. [Google Scholar] [CrossRef]
- Mazouria, S.E.; Aanniza, T.; Touhtouha, J.; Kandoussia, I.; Hakmia, M.; Belyamanib, L.; Ibrahimia, A.; Ouadghiria, M. Anthraquinones: A Promising Multi-target Therapeutic Scaffold To Treat COVID-19. Int. J. Appl. Biol. Pharm. 2021, 12, 338–355. [Google Scholar]
- Wald, A. Is chronic use of stimulant laxatives harmful to the colon? J. Clin. Gastroenterol. 2003, 36, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Katiyar, S.B.; Agarwal, A.; Chauhan, P.M. Perspective in antimalarial chemotherapy. Curr. Med. Chem. 2003, 10, 1137–1150. [Google Scholar] [CrossRef]
- Winter, R.W.; Cornell, K.A.; Johnson, L.L.; Ignatushchenko, M.; Hinrichs, D.J.; Riscoe, M.K. Potentiation of the antimalarial agent rufigallol. Antimicrob. Agents Chemother. 1996, 40, 1408–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, V.; Radaelli, M.; Straffi, L.; Rodegher, M.; Comi, G. Mitoxantrone: Benefits and risks in multiple sclerosis patients. Neurol. Sci. 2009, 30, 167–170. [Google Scholar] [CrossRef]
- Pickhardt, M.; Gazova, Z.; von Bergen, M.; Khlistunova, I.; Wang, Y.; Hascher, A.; Biernat, J.; Mandelkow, E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer’s paired helical filaments in vitro and in cells. J. Biol. Chem. 2005, 280, 3628–3635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Jin, H.; Sun, Q.R.; Xu, J.H.; Hu, H.T. Neuroprotective effects of emodin in rat cortical neurons against beta-amyloid-induced neurotoxicity. Brain Res. 2010, 1347, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, H.T.; Sun, Q.R. Neuroprotective effects of emodin on primary rat cortical neurons apoptosis induced by hydrogen peroxide. J. Chin. Med. Mater. 2010, 33, 1116–1119. [Google Scholar]
- Wang, C.; Zhang, D.; Ma, H.; Liu, J. Neuroprotective effects of emodin-8-O-beta-D-glucoside in vivo and in vitro. Eur. J. Pharmacol. 2007, 577, 58–63. [Google Scholar] [CrossRef]
- Jackson, T.C.; Verrier, J.D.; Kochanek, P.M. Anthraquinone-2-sulfonic acid (AQ2S) is a novel neurotherapeutic agent. Cell Death Dis. 2013, 4, e451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.G.; Kim, S.C.; Kim, Y.H.; Yang, W.S.; Kim, Y.; Hong, S.; Kim, K.H.; Yoo, B.C.; Kim, S.H.; Kim, J.H.; et al. Anti-Inflammatory and Antinociceptive Activities of Anthraquinone-2-Carboxylic Acid. Mediat. Inflamm. 2016, 2016, 1903849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Sanz, M.Á.; Poveda, J.L.; Montesinos, P. Daunorubicin and cytarabine for certain types of poor-prognosis acute myeloid leukemia: A systematic literature review. Expert Rev. Clin. Pharmacol. 2019, 12, 197–218. [Google Scholar] [CrossRef]
- Antolín, S.; Acea, B.; Albaina, L.; Concha, Á.; Santiago, P.; García-Caballero, T.; Mosquera, J.J.; Varela, J.R.; Soler, R.; Calvo, L. Primary systemic therapy in HER2-positive operable breast cancer using trastuzumab and chemotherapy: Efficacy data, cardiotoxicity and long-term follow-up in 142 patients diagnosed from 2005 to 2016 at a single institution. Breast Cancer 2019, 11, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Seetharam, M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr. Treat. Options Oncol. 2019, 20, 6. [Google Scholar] [CrossRef]
- Chen, W.; Liu, I.; Tomiyasu, H.; Lee, J.; Cheng, C.; Liao, A.T.; Liu, B.; Liu, C.; Lin, C. Imatinib enhances the anti-tumour effect of doxorubicin in canine B-cell lymphoma cell line. Vet. J. 2019, 254, 105398. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Asthana, S.; Gupta, P.; Dwivedi, P.D.; Tripathi, A.; Das, M. Toxicity of Naturally Occurring Anthraquinone. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 11, pp. 1–50. [Google Scholar]
- Barbosa, R.R.; Bourguignon, T.B.; Torres, L.D.; Arruda, L.S.; Jacques, T.M.; Serpa, R.G.; Calil, O.A.; Barbosa, L.F.M. Anthracycline-associated cardiotoxicity in adults: Systematic review on the cardioprotective role of beta-blockers. Rev. Assoc. Med. Bras. 2018, 64, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Volkova, M.; Raymond, R., 3rd. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.; Ruan, W.; Chen, Q. Understanding the Aqueous Solubility of Anthraquinone Sulfonate Salts: The Quest for High Capacity Electrolytes of Redox Flow Batteries. J. Electrochem. Soc. 2020, 167, 070522. [Google Scholar] [CrossRef]
- Fink, C.; Sun, D.; Wagner, K.; Schneider, M.; Bauer, H.; Dolgos, H.; Mäder, K.; Peters, S.A. Evaluating the Role of Solubility in Oral Absorption of Poorly Water-Soluble Drugs Using Physiologically-Based Pharmacokinetic Modeling. Clin. Pharmacol. Ther. 2020, 107, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Swiech, O.; Opuchlik, L.J.; Wojciuk, G.; Stepkowski, T.; Kruszewski, M.; Bilewicz, R. Doxorubicin carriers based on Au nanoparticles—Effect of shape and gold-drug linker on the carrier toxicity and therapeutic performance. RSC Adv. 2016, 6, 31960–31967. [Google Scholar] [CrossRef]
- Dzwonek, M.; Załubiniak, D.; Piątek, P.; Cichowicz, G.; Męczynska-Wielgosz, S.; Stępkowski, T.; Kruszewski, M.; Więckowska, A.; Bilewicz, R. Towards potent but less toxic nanopharmaceuticals—Lipoic acid bioconjugates of ultrasmall gold nanoparticles with an anticancer drug and addressing unit. RSC Adv. 2018, 8, 14947–14957. [Google Scholar] [CrossRef] [Green Version]
- Swiech, O.; Majdecki, M.; Debinski, A.; Krzak, A.; Bilewicz, R. Competition between self-inclusion and drug binding explains the pH dependence of the cyclodextrin drug carrier—Molecular modelling and electrochemistry studies. Nanoscale 2016, 8, 16733–16742. [Google Scholar] [CrossRef] [PubMed]
- Swiech, O.; Krzak, A.; Majdecki, M.; Trębińska-Stryjewska, A.; Wakuła, M.; Garbacz, P.; Gasiorowska, W.; Bilewicz, R. Water-soluble galactosamine derivative of β-cyclodextrin as protective ligand and targeted carrier for delivery of toxic anthracycline drug. Int. J. Pharm. 2020, 589, 119834. [Google Scholar] [CrossRef]
- Krzak, A.; Bilewicz, R. Voltammetric/UV–Vis study of temozolomide inclusion complexes with cyclodextrin derivatives. Bioelectrochemistry 2020, 136, 107587–107593. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Andou, K.; Fujiwara, S. Characterization and structural determination of 3A-amino-3A-deoxy-(2AS, 3AS)-cyclodextrins by NMR spectroscopy. Polym. J. 2012, 44, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–210. [Google Scholar]
- Swiech, O.; Dutkiewicz, P.; Wójciuk, K.; Chmurski, K.; Kruszewski, M.; Bilewicz, R. Cyclodextrin derivatives conjugated with aromatic moieties as pH-responsive drug carriers for anthracycline. J. Phys. Chem. B. 2013, 117, 13444–13450. [Google Scholar] [CrossRef]
- Sanli, S.; Altun, Y.; Guven, G. Solvent Effects on pKa Values of Some Anticancer Agents in Acetonitrile−Water Binary Mixtures. J. Chem. Eng. Data 2014, 59, 4015–4020. [Google Scholar] [CrossRef]
- Péan, C.; Djedaïni-Pilard, F.; Perly, B. Reliable NMR Experiments for the Determination of the Structure of Cyclodextrin Inclusion Complexes in Solution. In Proceedings of the Ninth International Symposium on Cyclodextrins, Santiago de Compostela, Spain, 31 May–3 June 1998; Labandeira, J.J.T., Vila-Jato, J.L., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 659–662. [Google Scholar]
- Wang, Z.; Landy, D.; Sizun, C.; Cézard, C.; Solgadi, A.; Przybylski, C.; de Chaisemartin, L.; Herfindal, L.; Barratt, G.; Legrand, F.X. Cyclodextrin complexation studies as the first step for repurposing of chlorpromazine. Int. J. Pharm. 2020, 584, 119391. [Google Scholar] [CrossRef]
- Mora, L.; Chumbimuni-Torres, K.Y.; Clawson, C.; Hernandez, L.; Zhang, L.; Wang, J. Real-time electrochemical monitoring of drug release from therapeutic nanoparticles. J. Control Release 2009, 140, 69–73. [Google Scholar] [CrossRef]
- Osa, T.; Matsue, T.; Fujihira, T. Cyclodextrin-nitrophenol system studied by polarography. Heterocycles 1977, 6, 1833–1839. [Google Scholar] [CrossRef]









| Water | |||
| Cyclodextrin | Solubility (a) increase (%) | K1:1 (M−1) | R2 |
| βCD | 128 | 460 ± 45 | 0.9911 |
| βCDamine | 77 | 280 ± 30 | 0.9900 |
| βCDLip | 238 | 800 ± 40 | 0.9880 |
| βCDGAL | 234 | 840 ± 45 | 0.9896 |
| Britton–Robinson Buffer pH 7.4 | |||
| Cyclodextrin | Solubility (b) increase (%) | K1:1 (M−1) | R2 |
| βCD | 60 | 350 ± 40 | 0.9913 |
| βCDamine | 38 | 160 ± 20 | 0.9906 |
| βCDLip | 108 | 940 ± 25 | 0.9961 |
| βCDGAL | 125 | 1300 ± 60 | 0.9932 |
| Britton–Robinson Buffer pH 3.0 | |||
| Cyclodextrin | Solubility (c) increase (%) | K1:1 (M−1) | R2 |
| βCD | 464 | 1480 ± 180 | 0.9965 |
| βCDamine | 683 | 2450 ± 200 | 0.9910 |
| βCDLip | 734 | 2520 ± 175 | 0.9940 |
| βCDGAL | 820 | 2760 ± 110 | 0.9945 |
| Complex | Association constant K1:1 (M−1) | |
|---|---|---|
| pH 7.4 | pH 3.0 | |
| AQ2CA–βCD | 315 ± 40 | 1360 ± 180 |
| AQ2CA–βCDamine | 800 ± 70 | 2860 ± 190 |
| AQ2CA–βCDLip | 935 ± 60 | 2680 ± 230 |
| AQ2CA–βCDGAL | 1250 ± 90 | 2700 ± 150 |
| AQ2S–βCD | 175 ± 30 | 840 ± 50 |
| AQ2S–βCDamine | 250 ± 45 | 3040 ± 190 |
| AQ2S–βCDLip | 910 ± 40 | 2085 ± 135 |
| AQ2S–βCDGAL | 1450 ± 60 | 2500 ± 120 |
| Complex | Association Constant K1:1 (M−1) | |
|---|---|---|
| pH 7.4 | pH 5.5 | |
| DNR–βCD | 970 ± 35 | 780 ± 30 |
| DNR–βCDTriazol | 11,000 ± 500 | 3000 ± 200 |
| DNR–βCDGAL | 84,000 ± 5500 | 37,200 ± 3200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzak, A.; Swiech, O.; Majdecki, M.; Garbacz, P.; Gwardys, P.; Bilewicz, R. Adjusting the Structure of β-Cyclodextrin to Improve Complexation of Anthraquinone-Derived Drugs. Molecules 2021, 26, 7205. https://doi.org/10.3390/molecules26237205
Krzak A, Swiech O, Majdecki M, Garbacz P, Gwardys P, Bilewicz R. Adjusting the Structure of β-Cyclodextrin to Improve Complexation of Anthraquinone-Derived Drugs. Molecules. 2021; 26(23):7205. https://doi.org/10.3390/molecules26237205
Chicago/Turabian StyleKrzak, Agata, Olga Swiech, Maciej Majdecki, Piotr Garbacz, Paulina Gwardys, and Renata Bilewicz. 2021. "Adjusting the Structure of β-Cyclodextrin to Improve Complexation of Anthraquinone-Derived Drugs" Molecules 26, no. 23: 7205. https://doi.org/10.3390/molecules26237205
APA StyleKrzak, A., Swiech, O., Majdecki, M., Garbacz, P., Gwardys, P., & Bilewicz, R. (2021). Adjusting the Structure of β-Cyclodextrin to Improve Complexation of Anthraquinone-Derived Drugs. Molecules, 26(23), 7205. https://doi.org/10.3390/molecules26237205