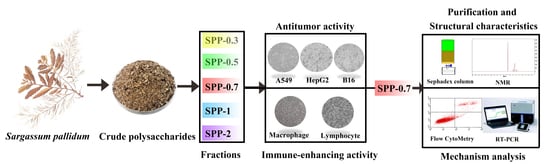
Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum
Abstract
:1. Introduction
2. Results
2.1. Purification and Chemical Characteristics of S. pallidum Polysaccharides
2.2. Fourier Transform Infrared (FT-IR) Spectrum Characterization
2.3. Antitumor Activity of Five Polysaccharide Fractions
2.4. Immunologic Modulation by the Five Polysaccharide Fractions
2.4.1. The Effect of the Five Polysaccharide Fractions on Macrophage (RAW264.7) Proliferation
2.4.2. The Effect of the Five Polysaccharide Fractions on Lymphocyte Proliferation
2.5. Purification and Nuclear Magnetic Resonance (NMR) Analysis of SPP-0.7
2.6. The Mechanism of the Antitumor and Immune-Enhancing Activities of SPP-0.7A
2.6.1. Apoptosis Analysis
2.6.2. Cytokine Expression Analysis
2.6.3. Transcriptomic Analysis of the Antitumor Mechanisms of SPP-0.7A
3. Discussion
4. Materials and Methods
4.1. Preparation of Crude Polysaccharide
4.2. Purification of Polysaccharides
4.3. Chemical Composition Analysis
4.4. FT-IR Spectrometry and NMR Analysis
4.5. Antitumor Activity Assay
4.5.1. Cell Lines and Culture
4.5.2. Cell Proliferation Assay
4.5.3. Apoptosis Assay
4.6. Immune-Enhancing Activity Assay
4.6.1. RAW264.7 Proliferation Assay
4.6.2. Mouse Lymphocyte Proliferation Assay
4.6.3. Assay of Cytokines Expression Level
4.7. Transcriptomic Assay and Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Zong, S.; Li, J.; Ye, Z.; Zhang, X.; Yang, L.; Chen, X.; Ye, M. Lachnum polysaccharide suppresses S180 sarcoma by boosting anti-tumor immune responses and skewing tumor-associated macrophages toward M1 phenotype. Int. J. Biol. Macromol. 2020, 144, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Starke, A.; Lindenmeyer, M.T.; Segerer, S.; Neusser, M.A.; Rüsi, B.; Schmid, D.M.; Cohen, C.D.; Wüthrich, R.P.; Fehr, T.; Waeckerle-Men, Y. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 2010, 78, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Gugel, E.; Saxena, M.; Bhardwaj, N. Modulation of innate immunity in the tumor microenvironment. Cancer Immunol. Immunother. 2016, 65, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Yang, R.; Yang, Y.; Wen, Y.; Liu, S.; Li, C.; Hu, Z.; Cheng, X.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Song, Z.; Li, Y.; Zhang, S.; Bao, J.; Wang, H.; Dong, C.; Ohizumi, Y.; Xu, J.; Guo, Y. Structural analysis and biological effects of a neutral polysaccharide from the fruits of Rosa laevigata. Carbohydr. Polym. 2021, 265, 118080. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yoon, Y.D.; Ahn, J.M.; Kang, J.S.; Park, S.-K.; Lee, K.; Song, K.B.; Kim, H.M.; Han, S.-B. Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. Int. Immunopharmacol. 2007, 7, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Chen, Y.; Nie, S.-P.; Xie, M.-Y.; He, M.; Zhang, S.-S.; Zhu, K.-X. Ganoderma atrum polysaccharide induces anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. J. Cell. Biochem. 2011, 112, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from Sargassum pallidum. Carbohydr. Polym. 2020, 239, 116230. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yuan, X.; Zeng, Y.; Nie, K.; Li, Z.; Wang, Z. Structure elucidation of a major fucopyranose-rich heteropolysaccharide (STP-II) from Sargassum thunbergii. Carbohydr. Polym. 2016, 143, 1–8. [Google Scholar] [CrossRef]
- Cao, Y.; Duan, J.A.; Fan, X.S.; Guo, J.M.; Shu-Lan, S.U. Exploration and Analysis of the Herbal Nature and Application Characteristics of Sargassum. Chin. J. Exp. Tradit. Med. Formulae 2014. [Google Scholar]
- Ma, Y.; Zhang, Y.; Zhai, Y.; Zhu, Z.; Pan, Y.; Qian, D.; Su, S.; Fan, X.; Duan, J. Development of a UPLC-TQ/MS Approach for the Determination of Eleven Bioactive Components in Haizao Yuhu Decoction Plus-Minus Haizao and Gancao Drug Combination after Oral Administration in a Rat Model of Hypothyroidism. Molecules 2016, 22, 7. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2017, 155, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, C.; Chen, Q.; Huang, Q.; Pérez, M.E.M.; Fu, X. Physicochemical characterization, potential antioxidant and hypoglycemic activity of polysaccharide from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 139, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fu, X.; Cao, C.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Mar. Drugs 2015, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Sivasankar, P.; Sivakumar, L.; Vairamani, S.; Shanmugam, A. Isolation and chemical characteristics of rhamnose enriched polysaccharide from Grateloupia lithophila. Carbohydr. Polym. 2018, 195, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Bao, A.; Liu, X.; Zeng, J.; Yang, X.; Yao, J.; Zhang, J.; Lei, Z. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: Solution conformation and anti-tumor activities in vitro. Carbohydr. Polym. 2016, 152, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Rochas, C.; Lahaye, M.; Yaphe, W. Sulfate Content of Carrageenan and Agar Determined by Infrared Spectroscopy. Bot. Mar. 1986, 29, 335–340. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Zhu, Z.-Y.; Sun, H.-Q.; Chen, L.-J. Structural characterization and inhibition on α-glucosidase activity of acidic polysaccharide from Annona squamosa. Carbohydr. Polym. 2017, 174, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.X.; Yang, J.F.; Ni, L.M.; Gao, Q.; Liu, Z.J. The chemical and structural transformation of bamboo wastes during torrefaction process. Environ. Prog. Sustain. Energy 2021, 40, 8. [Google Scholar] [CrossRef]
- Kuang, H.; Jiao, Y.; Wang, W.; Wang, F.; Chen, Q. Characterization and antioxidant activities of intracellular polysaccharides from Agaricus bitorquis (QuéL.) Sacc. Chaidam ZJU-CDMA-12. Int. J. Biol. Macromol. 2020, 156, 1112–1125. [Google Scholar] [CrossRef]
- El Rashed, Z.; Lupidi, G.; Kanaan, H.; Grasselli, E.; Canesi, L.; Khalifeh, H.; Demori, I. Antioxidant and Antisteatotic Activities of a New Fucoidan Extracted from Ferula hermonis Roots Harvested on Lebanese Mountains. Molecules 2021, 26, 1161. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ying, T. Isolation, molecular characterization and antioxidant activity of a water-soluble polysaccharide extracted from the fruiting body of Termitornyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 122, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Prieto, A.; Leal, J.A.; Jiménez-Barbero, J.; Bernabé, M. Fungal cell wall galactomannan isolated from Apodus deciduus. Carbohydr. Res. 2002, 337, 1503–1506. [Google Scholar] [CrossRef]
- Zych, K.; Toukach, F.V.; Arbatsky, N.P.; Kolodziejska, K.; Senchenkova, S.N.; Shashkov, A.S.; Knirel, Y.A.; Sidorczyk, Z. Structure of the O-specific polysaccharide of Proteus mirabilis D52 and typing of this strain to Proteus serogroup O33. Eur J. Biochem 2001, 268, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Shi, L.; Li, Y.; Tuerhong, M.; Abudukeremu, M.; Cui, J.; Li, Y.; Jin, D.-Q.; Xu, J.; et al. Structure features, selenylation modification, and improved anti-tumor activity of a polysaccharide from Eriobotrya japonica. Carbohydr. Polym. 2021, 118496. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C.; Zhu, Z.-Y.; Liu, Y.-L.; Sun, H.-Q. Comparisons of the anti-tumor activity of polysaccharides from fermented mycelia and cultivated fruiting bodies of Cordyceps militaris in vitro. Int. J. Biol. Macromol. 2019, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Arain, M.A.; Ali Fazlani, S.; Marghazani, I.B.; Umar, M.; Soomro, J.; Bhutto, Z.A.; Soomro, F.; Noreldin, A.E.; Abd El-Hack, M.E.; et al. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Jin, J.-O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yu, G.; Nie, W.; Jin, J.; Chen, L.; Chen, X. Antitumor activity and underlying mechanism of Sargassum fusiforme polysaccharides in CNE-bearing mice. Int. J. Biol. Macromol. 2018, 112, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhang, J.; Nie, W.; Zhou, W.; Jin, L.; Chen, X.; Lu, J. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef]
- Jiao, L.; Li, X.; Li, T.; Jiang, P.; Zhang, L.; Wu, M.; Zhang, L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009, 9, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Chen, C.; Li, C.; Huang, Q.; Fu, X. Physicochemical characterization, antioxidant and hypoglycemic activities of selenized polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 132, 308–315. [Google Scholar] [CrossRef]
- Cao, C.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, D.; Li, C.; Fu, X. Physicochemical properties and bioactivity of polysaccharides from Sargassum pallidum by fractional ethanol precipitation. Int. J. Food Sci. Technol. 2021, 56, 3536–3545. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, J.; Lu, C.; Yan, T.; Qian, Y.; Shen, H.; Zhao, Y.; Wang, J.; Kong, P.; Zhang, X. Systematic Transcriptome Analysis Reveals the Inhibitory Function of Cinnamaldehyde in Non-Small Cell Lung Cancer. Front. Pharmacol. 2021, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Jiang, S.; Mu, J.; Yu, H.; Duan, H.; Deng, X. Antitumor immunostimulatory activity of polysaccharides from Panax japonicus C. A. Mey: Roles of their effects on CD4+ T cells and tumor associated macrophages. Int. J. Biol. Macromol. 2018, 111, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Baugh, J.; Bucala, R. Mechanisms for modulating TNF alpha in immune and inflammatory disease. Curr. Opin. Drug Discov. Dev. 2001, 4, 635–650. [Google Scholar]
- Lejeune, F.J.; Liénard, D.; Matter, M.; Rüegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6. [Google Scholar] [PubMed]
- Chu, W.; Cao, L.; Daokun, G.; Zhao, J. iNOS Promotes the Development of Osteosarcoma via Wntβ-Catenin Pathway. J. Immunol. Res. 2021, 2021, 4549221. [Google Scholar] [CrossRef]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Jin, W.; Liu, B.; Li, S.; Chen, J.; Tang, H.; Jiang, D.; Zhang, Q.; Zhong, W. The structural features of the sulfated heteropolysaccharide (ST-1) from Sargassum thunbergii and its neuroprotective activities. Int. J. Biol. Macromol. 2018, 108, 307–313. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Alam, N.; Gupta, P.C. Structure of a water-soluble polysaccharide from the seeds of Cassia angustifolia. Planta Med. 1986, 4, 308–310. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, H.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 22–25. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L. Characterization of a heteropolysaccharide isolated from diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xiong, W.; Zeng, L.; Wang, D.; Liu, J.; Wu, Y.; Hu, Y. Comparison of Bush Sophora Root polysaccharide and its sulfate’s anti-duck hepatitis A virus activity and mechanism. Carbohydr. Polym. 2014, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.-T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Shen, Y.; Wang, J. Transcriptomic analysis of rice (Oryza sativa) endosperm using the RNA-Seq technique. Plant. Mol. Biol. 2013, 81, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An In Vitro Whole-Transcriptomic Approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, S.; Pohl, M.O.; Zhou, Y.; Rodriguez-Frandsen, A.; Wang, G.; Stein, D.A.; Moulton, H.M.; DeJesus, P.; Che, J.; Mulder, L.C.F.; et al. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe 2015, 18, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. 2), W316–W322. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Sample | Yield (%) | Total Sugar (%) | Sulfate (%) | Monosaccharides Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glc | Gal | xyl | Fuc | ||||
| SPP-0.3 | 11.90% | 43.20% | 5.39% | 0.110 | 1 | - | 0.759 | - | 0.384 | 0.131 |
| SPP-0.5 | 7.50% | 67.16% | 6.88% | 0.902 | 0.512 | - | 0.699 | 0.733 | 0.273 | 1 |
| SPP-0.7 | 5.90% | 73.27% | 9.57% | 0.671 | - | - | - | 0.217 | - | 1 |
| SPP-1 | 9.20% | 62.59% | 8.80% | - | 1 | - | 0.012 | 0.002 | 0.003 | - |
| SPP-2 | 2.30% | 63.45% | 13.79% | - | 1 | - | - | 0.052 | - | 0.006 |
| PathwayID | PathwayTerm | p-Value | Enrichment |
|---|---|---|---|
| PATH:05222 | Small cell lung cancer | 0.0038138 | 10.869984 |
| PATH:05200 | Pathways in cancer | 0.0064712 | 4.8860029 |
| PATH:05145 | Toxoplasmosis | 0.0115104 | 7.1661376 |
| PATH:04610 | Complement and coagulation cascades | 0.0225988 | 9.4845938 |
| PATH:05211 | Renal cell carcinoma | 0.0238089 | 9.2136054 |
| PATH:05212 | Pancreatic cancer | 0.0238089 | 9.2136054 |
| PATH:04622 | RIG-I-like receptor signaling pathway | 0.0244236 | 9.0838364 |
| PATH:05220 | Chronic myeloid leukemia | 0.0256719 | 8.8349641 |
| PATH:00980 | Metabolism of xenobiotics by cytochrome P450 | 0.0315934 | 7.8652729 |
| PATH:05410 | Hypertrophic cardiomyopathy (HCM) | 0.032281 | 7.7705106 |
| PATH:05414 | Dilated cardiomyopathy | 0.037253 | 7.1661376 |
| PATH:04210 | Apoptosis | 0.037253 | 7.1661376 |
| PATH:05205 | Proteoglycans in cancer | 0.0413872 | 4.2996825 |
| PATH:05142 | Chagas disease (American trypanosomiasis) | 0.0471805 | 6.2616736 |
| PathwayID | PathwayTerm | p-Value | Enrichment |
|---|---|---|---|
| PATH:00790 | Folate biosynthesis | 0.0015041 | 42.0621118 |
| PATH:04390 | Hippo signaling pathway | 0.0029298 | 7.697641375 |
| PATH:04350 | TGF-beta signaling pathway | 0.0043803 | 10.27098079 |
| PATH:05219 | Bladder cancer | 0.0110488 | 14.02070393 |
| PATH:04115 | p53 signaling pathway | 0.0264354 | 8.659846547 |
| PATH:05202 | Transcriptional misregulation in cancer | 0.0296241 | 4.907246377 |
| PATH:04260 | Cardiac muscle contraction | 0.0337453 | 7.54960981 |
| PATH:05410 | Hypertrophic cardiomyopathy (HCM) | 0.0376566 | 7.094814039 |
| PATH:05414 | Dilated cardiomyopathy | 0.0434007 | 6.542995169 |
| PATH:04015 | Rap1 signaling pathway | 0.0445293 | 4.146968769 |
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| IL-6 | F | CATGTTCTCTGGGAAATCGTGG |
| R | AACGCAACTAGGTTTGCCGAGTA | |
| IL-1β | F | GGGATGATGATGATAACCTG |
| R | TTGTCGTTGCTTGGTTCTCCT | |
| TNF-α | F | GATCTCAAAGCAAACCAACTAGTG |
| R | CTCCAGCTGGAAGACTCCCAG | |
| iNOS | F | GGTCTTCCTGGGCTCGATCTG |
| R | GCCGTGGCCAACATGCTACT | |
| β-actin | F | GCAGAAGGAGATCACTGCCCT |
| R | GCTGATCCACATCTGCTGGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. https://doi.org/10.3390/molecules26247559
Gao Y, Li Y, Niu Y, Ju H, Chen R, Li B, Song X, Song L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules. 2021; 26(24):7559. https://doi.org/10.3390/molecules26247559
Chicago/Turabian StyleGao, Yi, Yizhen Li, Yunze Niu, Hao Ju, Ran Chen, Bin Li, Xiyun Song, and Lin Song. 2021. "Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum" Molecules 26, no. 24: 7559. https://doi.org/10.3390/molecules26247559
APA StyleGao, Y., Li, Y., Niu, Y., Ju, H., Chen, R., Li, B., Song, X., & Song, L. (2021). Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules, 26(24), 7559. https://doi.org/10.3390/molecules26247559