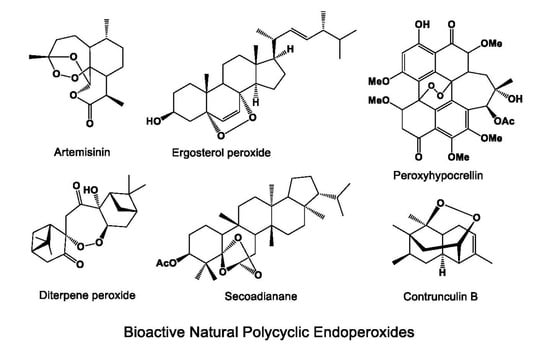
Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity
Abstract
:1. Introduction
2. Polycyclic Endoperoxides Derived from Marine Sources
3. Polycyclic Endoperoxides Derived from Fungi and Fungal Endophytes
4. Polycyclic Endoperoxides Derived from Plants and Liverworts
5. Comparison of Biological Activities of Natural Polycyclic Endoperoxides
5.1. Antiprotozoal Activity of Natural Polycyclic Endoperoxides
5.2. Antitumor and Other Activities of Natural Polycyclic Endoperoxides
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dembitsky, V.M. Bioactive peroxides as potential therapeutic agents. Eur. J. Med. Chem. 2008, 43, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Oxidation, epoxidation and sulfoxidation reactions catalysed by haloperoxidases. Tetrahedron 2003, 26, 4701–4720. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Chemistry and biodiversity of the biologically active natural glycosides. Chem. Biodivers 2004, 1, 673–781. [Google Scholar] [CrossRef] [PubMed]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1992, 9, 289–312. [Google Scholar] [CrossRef]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1999, 16, 55–73. [Google Scholar] [CrossRef]
- Siddiq, A.; Yaremenko, I.; Terent’v, A.O.; Gloriozova, T.A.; Dzhemileva, L.U.; D’yakonov, A.V.; Vil, V.; Dembitsky, V.M. Phytomedicinal aspects of sesquiterpenoid peroxides: Origin, structures and biological activity. Frontiers Drug Chem. Clin. Res. 2019, 2, 1–10. [Google Scholar]
- Sikorsky, T.V.; Ermolenko, E.V.; Gloriozova, T.A.; Dembitsky, V.M. Mini Review: Anticancer activity of diterpenoid peroxides. Vietnam J. Chem. 2020, 58, 273–280. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and unstable 1,2-dioxolanes: Origin, synthesis, and biological activities. Sci. Synth. Knowl. Updates 2020, 38, 277–321. [Google Scholar]
- Dembitsky, V.M.; Vil, V.A. Medicinal chemistry of stable and unstable 1,2-dioxetanes: Origin, formation, and biological activities. Sci. Synth. Knowl. Updates 2019, 38, 333–377. [Google Scholar]
- Noronha, M.; Pawar, V.; Prajapati, A.; Subramanian, R.B. A literature review on traditional herbal medicines for malaria. S. Afr. J. Bot. 2020, 128, 292–303. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.B.; Hu, L. Natural endoperoxides as drug lead compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7657–7667. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Terent’ev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Intern. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Dembitsky, V.; Shkrob, I.; Hanus, L.O. Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 209–215. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural peroxides as potential therapeutic agents. J. Mol. Genet. Med. 2015, 9, 1000163. [Google Scholar]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-Inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; Affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2020, 18, 5. [Google Scholar]
- Budde, S.; Goerdeler, F.; Floß, J.; Kreitmeier, P.; Hicks, E.F.; Moscovitz, O.; Seeberger, P.H.; Davies, H.M.L.; Reiser, O. Visible light mediated oxidative ring expansion of anellated cyclopropanes to fused endoperoxides with antimalarial activity. Org. Chem. Front. 2020, 7, 1789–1795. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Li, Y.; Zhang, X.; Wong, Y.K.; Shen, S.; Zhong, T.; Zhang, J.; Liu, Q.; Wang, J. Advances in the research on the targets of anti-malaria actions of artemisinin. Pharmacol. Ther. 2020, 216, 107697. [Google Scholar] [CrossRef] [PubMed]
- Tajuddeen, N.; Van Heerden, F.R. Anti-plasmodial natural products: An update. Malar. J. 2019, 18, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rukunga, G.; Simons, A.J. The Potential of Plants as a Source of Antimalarial Agents: A Review; Planta Phile Publications: Berlin, Germany, 2006; 72p. [Google Scholar]
- Tiwari, M.K.; Chaudhary, S. Artemisinin-derived antimalarial endoperoxides from bench-side to bed-side: Chronological advancements and future challenges. Med. Res. Rev. 2020, 40, 1220–1275. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D. Global prevalence and mortality of severe Plasmodium malariae infection: A systematic review and meta-analysis. Malar. J. 2020, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Plenderleith, L.J.; Hahn, B.H. Ape Origins of human malaria. Annu. Rev. Microbiol. 2020, 74, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. Malaria researchers wait for industry to join fight. Science 2000, 287, 1956–1958. [Google Scholar] [CrossRef] [PubMed]
- Chima, R.I.; Goodman, C.A.; Mills, A. The economic impact of malaria in Africa: A critical review of the evidence. Health Policy 2003, 63, 17–36. [Google Scholar] [CrossRef]
- Cock, I.E.; Selesho, M.I.; van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of malaria. J. Ethnopharm. 2019, 245, 112176. [Google Scholar] [CrossRef]
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2020, 14, 216–227. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Druzhilovskiy, D.S.; Rudik, A.V.; Stolbov, L.A.; Dmitriev, A.V.; Tarasova, O.A.; Ivanov, S.M.; et al. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.; Filimonov, D.; Poroikov, V.; Oprea, T.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR Without Borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological activities of epithio steroids. J. Pharm. Res. Intern. 2017, 18, 1–19. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2020, 529, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Bioactive cyclobutane-containing alkaloids. J. Nat. Med. 2008, 62, 1–33. [Google Scholar] [CrossRef]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 4. Fatty acid amide glycosides, their analogs and derivatives. Lipids 2005, 40, 641–660. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 6. Biologically active marine and terrestrial alkaloid glycosides. Lipids 2005, 40, 1081–1114. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 7. Biologically active hemi-and monoterpenoid glycosides. Lipids 2006, 41, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids 2005, 40, 535–557. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 1. Glycosides of fatty acids and alcohols. Lipids 2004, 39, 933–953. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 2. Polyether glycosidic ionophores and macrocyclic glycosides. Lipids 2005, 40, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Temina, M.; Tolstikov, A.G.; Dembitsky, V.M. Natural microbial UV radiation filters - mycosporine-like amino acids. Folia Microbiol. 2004, 49, 339–352. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Chemistry, origin, antitumor and other activities of fungal homo-dimeric alkaloids. Mathews J. Pharmaceut. Sci. 2016, 1, 004. [Google Scholar]
- Kilimnik, A.; Kuklev, D.V.; Dembitsky, V.M. Antitumor Acetylenic Lipids. Mathews J. Pharmaceut. Sci. 2016, 1, 005. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Novel antitumor agents: Marine sponge alkaloids, their synthetic analogs and derivatives. Mini Rev. Med. Chem. 2005, 5, 319–336. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 1996, 35, 1–51. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Ali, H.A.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O. Arsenolipids. Prog. Lipid Res. 2004, 43, 403–448. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A.; Pechenkina, E.E. Glycolipids, phospholipids and fatty acids of brown algae species. Phytochemistry 1990, 29, 3417–3421. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Pechenkina-Shubina, E.E.; Rozentsvet, O.A. Glycolipids and fatty acids of some seaweeds and marine grasses from the Black Sea. Phytochemistry 1991, 30, 2279–2283. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Řezanka, T. Metabolites produced by nitrogen fixing Nostoc species. Folia Microbiol. 2005, 50, 363–391. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bromo- and iodo-containing alkaloids from marine microorganisms and sponges. Russ. J. Bioorg. Chem. 2002, 28, 170–182. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Plasmalogens in phospholipids of marine invertebrates. Russ. J. Mar. Biol. 1979, 5, 86–90. [Google Scholar]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rozentsvet, O.A. Diacylglyceryltrimethylhomoserines and phospholipids of some green marine macrophytes. Phytochemistry 1989, 28, 3341–3343. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Natural neo acids and neo alkanes: Their analogs and derivatives. Lipids 2006, 41, 309–340. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Rozentsvet, O.A. Phospholipid composition of some marine red algae. Phytochemistry 1990, 29, 3149–3152. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Trunculin-F and contrunculin-A and -B: Novel oxygenated norterpenes from a southern Australian marine sponge, Latrunculia conulosa. Aust. J. Chem. 1993, 46, 1363–1374. [Google Scholar] [CrossRef]
- Ovenden, S.P.; Capon, R.J. Trunculins G–I: New norsesterterpene cyclic peroxides from a southern Australian marine sponge, Latrunculia sp. Aust. J. Chem. 1998, 51, 573–580. [Google Scholar] [CrossRef]
- Hirade, H.; de Voogd, N.J.; Suzuka, T.; Tanaka, J. Trunculins X and Y from an Okinawan sponge Sigmosceptrella sp. Tetrahedron 2019, 75, 4620–4625. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Martin, J.D.; Perez, C.; Rovirosa, J.; Tagle, B.; Clardy, J. Isolation and X-ray structural determination of three new diterpenoids from the marine alga Taonia atomaria. Chem. Lett. 1984, 13, 1649–1652. [Google Scholar] [CrossRef]
- Kusumi, T.; Ohtani, I.; Inouye, Y.; Kakisawa, I. Absolute configurations of cytotoxic marine cembranolides; Consideration of mosher’s method. Tetrahedron Lett. 1988, 29, 4731–4734. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Kuramoto, J.; Nakayama, M.; Hase, T. Denticulatolide, an ichthyotoxic peroxidecontaining cembranolide from the soft coral Lobophytum denticulatum. Tetrahedron Lett. 1985, 26, 4487–4490. [Google Scholar] [CrossRef]
- Fukazawa, Y. Conformational study of the cembranolide diterpene denticulatolide by molecular mechanics method. Tetrahedron Lett. 1986, 27, 1825–1828. [Google Scholar] [CrossRef]
- Uchio, Y.; Shizuko, E.; Yoshimasa, F.; Mitsuaki, K. 7-Epidenticulatolide, a new cembranolide with a cyclic peroxide function from the soft coral Lobophytum denticulatum. Bull. Chem. Soc. Japan 1992, 65, 1182–1185. [Google Scholar] [CrossRef]
- Hambley, T.W.; Taylor, W.C.; Toth, S. The constituents of marine sponges. IX New norditerpenoids from Aplysilla pallida. Aust. J. Chem. 1997, 50, 903–910. [Google Scholar] [CrossRef]
- Monaco, P.; Parrilli, M.; Previtera, L. Two endoperoxide diterpenes from Elodea Canadensis. Tetrahedron Lett. 1987, 28, 4609–4612. [Google Scholar] [CrossRef]
- Hirota, H.; Okino, T.; Yoshimura, E.; Fusetani, N. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron 1998, 54, 13971–13980. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Kuo, Y.-H.; Dai, C.-F.; Sheu, J.H. Oxygenated terpenoids from a Formosan Soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 1208–1212. [Google Scholar] [CrossRef]
- Sera, Y.; Adachi, K.; Shizuri, Y. A new epidioxy sterol as an antifouling substance from a Palauan marine sponge, Lendenfeldia chondrodes. J. Nat. Prod. 1999, 62, 152–154. [Google Scholar] [CrossRef]
- Seo, Y.W.; Rho, J.R.; Cho, K.W.; Sim, C.J.; Shin, J.H. Isolation of epidioxysteroids from a sponge of the genus Tethya. Bull. Korean Chem. Soc. 1997, 18, 631–635. [Google Scholar]
- Aknin, M.; Viracaoundin, I.; Faure, R.; Gaydou, E.M. 5α,8α-Epidioxycholest-6-en-3-β-ol from three cone snails of the Indian ocean. J. Am. Oil Chem. Soc. 1998, 75, 1679–1681. [Google Scholar] [CrossRef]
- Seo, Y.; Rho, J.R.; Shin, J. Isolation of two steroids from the marine polychaete worm Perinersis aibuhitensis. Ocean Res. 1996, 18, 83–87. [Google Scholar]
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Sterol peroxides from the sponge Axinella cannabina. Gazz. Chim. Gazz. Ital. 1974, 104, 409–413. [Google Scholar]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E.M. Isolation of bioactive 5α,8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000, 78, 986–992. [Google Scholar] [CrossRef]
- Abourriche, A.; Charrouf, M.; Chaib, N.; Bennamara, A.; Bontemps, N.; Francisco, C. Isolation and bioactivities of epidioxysterol from the tunicate Cynthia savignyi. Farmaco 2000, 55, 492–494. [Google Scholar] [CrossRef]
- Minh, C.V.; Van Kiem, P.; Kim, Y.H. Cytotoxic constituents of Diadema setosum. Arch. Pharmacal. Res. 2004, 27, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5α,8α-epidoxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Jimenez, C.; Quiño, A.E.; Do Caste, L.; Riguera, R. Epidioxy sterols from the tunicates Dendrodoa grossularia and Ascidiella aspersa and the gastropoda Aplysia depilans and Aplysia punctate. J. Nat. Prod. 1986, 49, 905–909. [Google Scholar] [CrossRef]
- Findlay, J.A.; Patil, A.D. A novel sterol peroxide from the sea anenome Metridium senile. Steroids 1984, 44, 261–265. [Google Scholar] [CrossRef]
- Sheikh, Y.M.; Djerassi, C. Steroids from sponges. Tetrahedron 1974, 30, 4095–4103. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Sagar, K.S.; Venugopal, M.J.R.V. Terpenoid and steroid constituents of the Indian ocean soft coral Sinularia maxima. Tetrahedron 1995, 51, 10997–11010. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1997, 14, 257–302. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1991, 8, 97–114. [Google Scholar] [CrossRef]
- Toume, K.; Ishibashi, M. 5α,8α-Epidioxysterol sulfate from a diatom Odontella aurita. Phytochemistry 2002, 61, 359–360. [Google Scholar] [CrossRef]
- Yaoita, Y.; Amemiya, K.; Ohnuma, H.; Furumura, K.; Masaki, A.; Matsuki, T.; Kikuchi, M. Sterol constituents from five edible mushrooms. Chem. Pharm. Bull. 1998, 46, 944–950. [Google Scholar] [CrossRef] [Green Version]
- Stonard, R.J.; Petrovich, J.C.; Andersen, R.J. A new C26 sterol peroxide from the opisthobranch mollusk Adalaria sp. and the sea pen Virgularia sp. Steroids 1980, 36, 81–86. [Google Scholar] [CrossRef]
- Prawat, H.; Mahidol, C.; Wittayalai, S.; Intachote, P.; Kanchanapoom, T.; Ruchirawat, S. Nitrogenous sesquiterpenes from the Thai marine sponge Halichondria sp. Tetrahedron 2011, 67, 5651–5655. [Google Scholar] [CrossRef]
- Mishra, P.D.; Wahidullah, S.; De Souza, L.; Kamat, S.Y. Lipid constituents of marine sponge Suberites carnosus. Indian J. Chem. 1996, 35, 806–809. [Google Scholar]
- Im, K.S.; Nam, K.I.; Sim, C.J.; Jung, J.H. Sterol peroxide derivatives from the marine sponge Spirastrella abata. Korean J. Pharmacog. 2000, 31, 401–406. [Google Scholar]
- Feng, Y.; Khokhara, S.; Davis, R.A. Crinoids: Ancient organisms, modern chemistry. Nat. Prod. Rep. 2017, 34, 571–584. [Google Scholar] [CrossRef]
- Mun, B.; Wang, W.; Kim, H. Cytotoxic 5α,8α-epidioxy sterols from the marine sponge Monanchora sp. Arch. Pharm. Res. 2015, 38, 18–25. [Google Scholar] [CrossRef]
- Ioannou, E.; Aazika, A.F.A.; Dimitrios, M.Z.; Xanthippi, C.; Constantinos, A.; Alexisd, V.M.N.; Roussisa, V. 5α,8α-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids 2009, 74, 73–80. [Google Scholar] [CrossRef]
- Dembitsky, V.M. The multiple properties of some of the lichenized Ascomycetes: Biological activity and active metabolites. In Plant Adaptation Strategies in Changing Environment; Shukla, V., Kumar, S., Kumar, N., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Torres, A.; Hochberg, M.; Pergament, I.; Smoum, R.; Niddam, V.; Dembitsky, V.M. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur. J. Biochem. 2004, 271, 780–784. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Řezanka, T.; Spížek, J.; Hanuš, L.O. Secondary metabolites of slime molds (myxomycetes). Phytochemistry 2005, 66, 747–769. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Lipids of lichens. Prog. Lipid Res. 1992, 31, 373–397. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A.; Shustov, M.V. Identification of fatty acids from Cladonia lichens. Phytochemistry 1991, 30, 4015–4018. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A.; Shustov, M.V. Fatty acid composition of Parmelia lichens. Phytochemistry 1992, 31, 841–843. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A. Fatty acids and phospholipids from lichens of the order Lecanorales. Phytochemistry 1992, 31, 851–853. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Shubina, E.E.; Kashin, A.G. Phospholipid and fatty acid composition of some Basidiomycetes. Phytochemistry 1992, 31, 845–849. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Terent’ev, A.O.; Levitsky, D.O. Amino and fatty acids of wild edible mushrooms of the genus Boletus. Records Nat. Prod. 2010, 4, 218–225. [Google Scholar]
- Rustamova, N.; Bozorov, K.; Efferth, T. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. PhytoChem. Rev. 2020, 19, 425–448. [Google Scholar] [CrossRef]
- Vil, V.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Naturally occurring of α,β-diepoxy-containing compounds: Origin, structures, and biological activities. Appl. Microbiol. Biotech. 2019, 103, 3249–3264. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Ming, Q.; Wu, L.; Rahman, K.; Qin, L. Alkaloids produced by endophytic fungi: A review. Nat. Prod. Commun. 2012, 7, 963–968. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Gödecke, T.; Gunn, J.; Duan, J.A.; Che, C.T. Protostane and fusidane triterpenes: A mini review. Molecules 2013, 18, 4054–4080. [Google Scholar] [CrossRef] [PubMed]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Trung, H.V.; Tuan, N.N.; Thanh, N.T.; Giang, T.T.B.; Giang, D.T.T.; Ogunwande, I.; Thang, T.D. Determination of ergosterol and ergosterol peroxide in higher fungi species by high-performance liquid chromatography. J. Pharm. Phytochem. 2018, 7, 2376–2379. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T.; Shubina, E.E. Unusual hydroxy fatty acids from some higher fungi. Phytochemistry 1993, 34, 1057–1059. [Google Scholar] [CrossRef]
- Mallavadhani, U.U.; Sudhakar, A.V.S.; Satyanarayana, K.V.S.; Mahapatraa, A.; Li, W. Chemical and analytical screening of some edible mushrooms. Food. Chem. 2006, 95, 58–64. [Google Scholar] [CrossRef]
- Ragasa, C.Y. Anticancer compounds from nine commercially grown and wild Philippine mushrooms. Manila J. Sci. 2018, 11, 42–57. [Google Scholar]
- Kahlos, K.; Kangas, L.; Hiltunen, R. Ergosterol peroxide, an active compound from Inonotus radiatus. Planta Med. 1989, 55, 389–390. [Google Scholar] [CrossRef]
- Chen, Y.K.; Kuo, Y.H.; Chiang, B.H.; Lo, J.M.; Sheen, L.Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Cardile, V.; Piovano, M.; Caggia, S.; Espinoza, C.L.; Garbarino, J.A. Pro-apoptotic activity of ergosterol peroxide and (22E)-ergosta-7,22-dien-5α-hydroxy-3,6-dione in human prostate cancer cells. Chem. Biol. Interact. 2010, 184, 352–358. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Bu, M.; Hu, L.; Du, W.W.; Jiao, C.; Pan, H.; Sdiri, M.; Wu, N.; Xie, Y. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 2016, 7, 33948–33959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, K.; Aoki, T.; Takido, M.; Ikekawa, T.; Saito, H.; Matsuzawa, T. Inhibitory effects of ergosterol isolated from the edible mushroom Hypsizigus marmoreus on TPA-induced inflammatory ear oedema and tumour promotion in mice. Phytother. Res. 1994, 8, 10–13. [Google Scholar] [CrossRef]
- Bu, M.; Cao, T.; Li, H.; Guo, M.; Yang, B.B.; Zeng, C.; Hu, L. Synthesis of 5α,8α-ergosterol peroxide 3-carbamate derivatives and a fluorescent mitochondria-targeting conjugate for enhanced anticancer activities. ChemMedChem 2017, 12, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Cao, T.; Li, H.; Guo, M.; Yang, B.B.; Zeng, C.; Zhou, Y.; Zhang, N.; Hu, L. Synthesis and biological evaluation of novel steroidal 5α,8α-epidioxyandrost-6-ene-3β-ol-17-(O-phenylacetamide)oxime derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 3856–3861. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Govindharaj, M.; Arumugam, S.; Nirmala, G.; Bharadwaj, M.; Murugiyan, K. Effect of marine basidiomycetes fulvifomes sp.-derived ergosterol peroxide on cytotoxicity and apoptosis induction in MCF-7 Cell Line. J. Fungi 2019, 5, 16. [Google Scholar] [CrossRef]
- Serebryakov, E.P.; Simolin, A.V.; Kucherov, V.F.; Rosynov, B.V. New metabolites of Fusarium moniliforme sheld. Tetrahedron 1970, 26, 5215–5219. [Google Scholar] [CrossRef]
- Zang, M.; Ying, J.Z. Economic fungi in the South West of China; Scientific Press: Beijing, China, 1994. [Google Scholar]
- Yue, J.M.; Chen, S.N.; Lin, Z.W.; Sun, H.D. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 56, 801–806. [Google Scholar] [CrossRef]
- Miao, F.P.; Li, X.D.; Liu, X.H.; Cichewicz, R.H.; Ji, N.Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar Drugs 2012, 10, 131–139. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, T.; Xiang, X.; Gu, Q. Sterol composition in field-grown and cultured mycelia of Inonotus obliquus. Yaoxue Xuebao 2007, 42, 750–756. [Google Scholar]
- Zuo, W.; Luo, D.Q. Research on the chemical components of the fruit bodies of Boletus calopus. Anhui Nongye Kexue 2010, 38, 2356–2357. [Google Scholar]
- Yaoita, Y.; Matsuki, K.; Iijima, T.; Nakano, S.; Kakuda, R.; Machida, K.; Kikuchi, M. New sterols and triterpenoids from four edible mushrooms. Chem. Pharm. Bull. 2001, 49, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Li, X.M.; Li, C.S.; Wang, B.G. Nigerasterols A and B, antiproliferative sterols from the mangrovederived endophytic fungus Aspergillus niger MA-132. Helv. Chim. Acta 2013, 96, 1055–1061. [Google Scholar] [CrossRef]
- Yaoita, Y.; Endo, K.; Tani, Y.; Machida, K.; Amemiya, K. Sterol constituents from seven mushrooms. Chem. Pharm. Bull. 1999, 47, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Z.; Luo, M.H. Two new chamigrane metabolites from fermentation broth of Steccherinum ochraceum. Fitoterapia 2010, 81, 1205–1207. [Google Scholar] [CrossRef]
- Liu, D.Z.; Dong, Z.J.; Wang, F.; Liu, J.K. Two novel norsesquiterpene peroxides from basidiomycete Steccherinum ochraceum. Tetrahedron Lett. 2010, 51, 3152–3153. [Google Scholar] [CrossRef]
- Li, L.B.; Ren, J.; Lai, R.; Cheng, Z.M.; Zhu, H.J. Natural cyclic peroxide echinobithiophene A with antimicrobial activity from Echinops ritro L. Chem. J. Chinese Univ. 2011, 32, 891–896. [Google Scholar]
- Rahaman, M.S.; Siraj, M.A.; Sultana, S.; Seidel, V.; Islam, M.A. Molecular phylogenetics and biological potential of fungal endophytes from plants of the sundarbans Mangrove. Front. Microbiol. 2020, 11, 570855. [Google Scholar] [CrossRef]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef]
- She, Z.; Li, H.; Li, M.; Zhu, X.; Lin, Y. Norsesquiterpenoid Peroxide with Antitumor Activity and Preparation and Application Thereof. Chinese Patent CN 2011-10031487, 27 December 2011. Faming Zhuanli Shenqing. [Google Scholar]
- Li, Y.; Niu, S.; Sun, B.; Liu, S.; Liu, X. Cytosporolides A-C, antimicrobial meroterpenoids with a unique peroxylactone skeleton from Cytospora sp. Org. Lett. 2010, 12, 3144–3147. [Google Scholar] [CrossRef]
- Linington, R.; Navarro, G.; Pudhom, K.; McKerrow, J. Novel Semisynthetic Small Molecules for the Treatment Parasitic Disease. Patent WO 2012-US48743, 7 February 2013. PCT Int. Appl.. [Google Scholar]
- Chokpaiboon, S.; Sommit, D.; Teerawatananond, T.; Muangsin, N.; Bunyapaiboonsri, T.; Pudhom, K. Cytotoxic nor-chamigrane and chamigrane endoperoxides from a Basidiomycetous fungus. J. Nat. Prod. 2010, 73, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.T.J.; George, J.H. Structural reassignment of cytosporolides A–C via biomimetic synthetic studies and reinterpretation of NMR data. Org. Lett. 2011, 13, 5318–5321. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Niu, S.; Lu, X.; Chen, X.; Zhang, H. Unique metabolites of Pestalotiopsis fici suggest a biosynthetic hypothesis involving a Diels-Alder reaction and then mechanistic diversification. Chem. Commun. 2010, 46, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Chen, Y.-T.; Wan, X.-Y.; Edmund, F.; Puff, H.; Breitmaier, E. Die Struktur des Hypocrellins und seines Photooxidationsproduktes Peroxyhypocrellin. Liebigs Ann. Chem. 1981, 10, 1880–1885. [Google Scholar]
- Dembitsky, V.M. Bioactive fungal endoperoxides. Med Mycol. 2015, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Z.; Liu, J.K. Peroxy natural products. Nat. Prod. Bioprospect. 2013, 3, 161–206. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Therapeut. 2020, 216, 107658. [Google Scholar] [CrossRef]
- Liu, K.; Zuo, H.; Li, G.; Yu, H.; Hu, Y. Global research on artemisinin and its derivatives: Perspectives from patents. Pharmacol. Res. 2020, 159, 105048. [Google Scholar] [CrossRef]
- Czechowski, T.; Weathers, P.J.; Brodelius, P.E.; Brown, G.D.; Graham, I.A. Editorial: Artemisinin–from traditional Chinese medicine to artemisinin combination therapies; Four decades of research on the biochemistry, physiology, and breeding of Artemisia annua. Front. Plant Sci. 2020, 11, 594565. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Q. A new peroxide-sesquetepene: Nardosaldehyde. Nat. Prod. Res. Develop. 1997, 9, 7–9. [Google Scholar]
- Jayachandran, K.; Sekar, I.; Parthiban, K.T.; Amirtham, D.; Suresh, K.K. Analysis of different grades of agarwood (Aquilaria malaccensis Lamk.) oil through GC-MS. Indian J. Nat. Prod. Res. 2014, 5, 44–47. [Google Scholar]
- Caniato, R.; Puricelli, L. Review: Natural antimalarial agents (1995–2001). Crit. Rev. Plant Sci. 2003, 22, 79–105. [Google Scholar] [CrossRef]
- Takaya, Y.; Kurumada, K.I.; Takeuji, Y.; Kim, H.H.; Shibata, Y.; Ikemoto, N.; Wataya, Y.; Oshima, Y. Novel antimalarial guaiane-type sesquiterpenoids from Nardostachys chinensis roots. Tetrahedron Lett. 1998, 39, 1361–1364. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dutta, U.; Bandyopadhyay, D.; Nayak, A.; Basak, B.; Banerji, A.; Banerji, J. An overview of the genus Nardostachys. Nat. Prod. Commun. 2007, 2, 1163–1173. [Google Scholar] [CrossRef]
- Takaya, Y.; Takeuji, Y.; Akasaka, M.; Nakagawasai, O.; Tadano, T.; Kisara, K.; Kim, H.S.; Wataya, Y.; Niwa, M.; Oshima, Y. Novel guaiane endoperoxides, nardoguaianone A–D, from Nardostachys chinensis roots and their antinociceptive and antimalarial activities. Tetrahedron 2000, 56, 7673–7678. [Google Scholar] [CrossRef]
- Poornima, B.; Siva, B.; Shankaraiah, G.; Venkanna, A.; Nayak, V.L.; Ramakrishna, S.; Rao, C.V.; Babu, K.B. Novel sesquiterpenes from Schisandra grandiflora: Isolation, cytotoxic activity and synthesis of their triazole derivatives using “click” reaction. Eur. J. Med. Chem. 2015, 92, 449–458. [Google Scholar] [CrossRef]
- Dong, J.Y.; Ma, X.Y.; Cai, X.Q.; Yan, P.C.; Yue, L.; Lin, C. Sesquiterpenoids from Curcuma wenyujin with anti-influenza viral activities. Phytochemistry 2013, 85, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.M. Discovery of New Scaffolds for GABA (A) Receptor Modulators from Natural Origin. Ph.D. Thesis, Universität Basel, Basel, Switzerland, 2011. [Google Scholar]
- Sy, L.K.; Brown, G.D. Labdane diterpenoids from Alpinia chinensis. J. Nat. Prod. 1997, 60, 904–908. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, G.W.; Niu, X.M.; Li, W.Q.; Wang, F.S.; Li, S.H. Terpenes from Eupatorium adenophorum and their allelopathic effects on Arabidopsis seeds germination. J. Agric. Food Chem. 2009, 57, 478–482. [Google Scholar] [CrossRef]
- Loyola, L.A.; Morales, G.; Rodriguez, B.; Jiménez-Barbero, J.; De La Torre, M.C.; Perales, A.; Torres, M.R. Mulinic and isomulinic acids. Rearranged diterpenes with a new carbon skeleton from Mulinum crassifolium. Tetrahedron 1990, 46, 5413–5420. [Google Scholar] [CrossRef]
- Loyola, L.A.; Morales, G.; De La Torre, M.C.; Pedreros, S.; Rodríguez, B. 17-Acetoxymulinic acid, a rearranged diterpenoid from Mulinum crassifolium. Phytochemistry 1990, 29, 3950–3951. [Google Scholar] [CrossRef]
- Rojas-Alvareza, F.; Campos-Brionesa, C.; Lima, C.; Pérez, E.G.; Sepúlveda, B. Further mulinane diterpenoids from Azorella compacta Carlos Arechea. J. Pharm. Pharmacol. 2013, 65, 1231–1238. [Google Scholar]
- de Jesús Dzul-Beh, A.; Uc-Cachón, A.H.; Bórquez, J.; Loyola, L.A.; Peña-Rodríguez, L.M.; Molina-Salinas, G.M. Mulinane- and azorellane-type diterpenoids: A systematic review of their biosynthesis. Chem. Pharm. Biomol. 2020, 10, 1333. [Google Scholar]
- Itokawa, H.; Tachi, Y.; Kamano, Y.; Iitaka, Y. Structure of gilvanol, a new triterpene isolated from Quercus gilva Bume. Chem. Phar. Bull. 1978, 26, 331–333. [Google Scholar] [CrossRef] [Green Version]
- Itokawa, H.; Morita, H.; Katou, I.; Takeya, K.; Cavalheiro, A.J.; de Oliveira, R.C.B.; Ishige, M.; Motidome, M. Cytotoxic diterpenes from the rhizomes of Hedychium coronarium. Planta Med. 1988, 54, 311–315. [Google Scholar] [CrossRef]
- Kamchonwongpaisan, S.; Nilanonta, C.; Tarnchompoo, B.; Thebtaranonth, C.; Thebtaranonth, Y.; Yuthavong, Y.; Kongsaeree, P.; Clardy, J. An antimalarial peroxide from Amomum krervanh Pierre. Tetrahedron Lett. 1995, 36, 1821–1824. [Google Scholar] [CrossRef]
- Qinghaosu Antimalaria Coordinating Research Group. Antimalaria studies on qinghaosu. Chin. Med. J. 1979, 92, 811–816. [Google Scholar]
- Balint, G.A. Artemisinin and its derivatives: An important new class of antimalarial agents. Pharmacol. Therapeut. 2001, 90, 261–265. [Google Scholar] [CrossRef]
- McIntosh, H.; Olliaro, P. Cochrane infectious diseases group. Artemisinin derivatives for treating severe malaria. Cochrane Database Syst. Rev. 1998, 3, CD000527. [Google Scholar]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotech. 2012, 18, 247597. [Google Scholar] [CrossRef]
- Kiani, B.H.; Kayani, W.K.; Khayam, A.U.; Dilshad, E.; Ismail, H.; Mirza, B. Artemisinin and its derivatives: A promising cancer therapy. Mol. Biol. Rep. 2020, 47, 6321–6336. [Google Scholar] [CrossRef] [PubMed]
- Carolino, K.; Winzeler, E.A. The antimalarial resistome–finding new drug targets and their modes of action. Current Opin. Microbiol. 2020, 57, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sun, Z.; Kong, F.; Xiao, J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020, 188, 112044. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, P.H.; Krogstad, D.J.; Herwaldt, B.L. Antimalarial agents: Mechanisms of action. Antimicrob. Agents Chemther. 1988, 12, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [PubMed] [Green Version]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1–26. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Antifungals, antimalarials, and antivirals. Chem. Antibiot. Relat. Drugs 2016, 12, 175–195. [Google Scholar]
- Nagashima, F.; Suzuki, M.; Takaoka, S.; Asakawa, Y. New sesqui- and diterpenoids from the Japanese liverwort Jungermannia infusca (Mitt.) Sterh. Chem. Pharm. Bull. 1998, 46, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, F.; Suzuki, M.; Takaoka, S.; Asakawa, Y. New acorane-and cuparane-type sesqui-and new labdane-and seco-labdanne-type diterpenoids from the Japanese liverwort Jungermannia infusca (Mitt.) Steph. Tetrahedron 1999, 55, 9117–9121. [Google Scholar] [CrossRef]
- Chokpaiboon, S.; Sommit, D.; Bunyapaiboonsri, T.; Matsubara, K.; Pudhom, K. Antiangiogenic effect of chamigrane endoperoxides from a Thai mangrove-derived fungus. J. Nat. Prod. 2011, 74, 2290–2299. [Google Scholar] [CrossRef]
- Chen, H.J.; Wu, Y. Expeditious entry to the chamigrane endoperoxide family of natural products. Org. Lett. 2015, 17, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Ngo, K.S.; Brown, G.D. Allohimachalane, seco-allohimachalane and himachalane sesquiterpenes from Illicium tsangii. Tetrahedron 1999, 55, 759–766. [Google Scholar] [CrossRef]
- Ngo, K.S.; Brown, G.D. Santalane and isocampherenane sesquiterpenoids from Illicium tsangii. Phytochemistry 1999, 50, 1213–1219. [Google Scholar] [CrossRef]
- Ngo, K.S.; Wong, W.T.; Brown, G.D. Muurolane sesquiterpenes from Illicium tsangii. J. Nat. Prod. 1999, 62, 549–556. [Google Scholar] [CrossRef]
- Ma, W.H.; Tan, C.M.; He, J.C.; Duan, P.S.; Qin, L.P. A novel eudesmene sesquiterpenoid from Schisandra sphenanthera stems. Chem. Nat. Comp. 2011, 47, 713–717. [Google Scholar] [CrossRef]
- Adio, A.M.; König, W.A. Sesquiterpene constituents from the essential oil of the liverwort Plagiochila asplenioides. Phytochemistry 2005, 66, 599–609. [Google Scholar] [CrossRef]
- Nagashima, F.; Matsumura, N.; Ashigaki, Y.; Asakawa, Y. Chemical constituents of the liverworts Bryopteris filicina, Plagiochila asplenioides and Porella canariensis. J. Hattori Bot. Lab. 2003, 94, 197–204. [Google Scholar]
- Kundu, A.; Saha, S.; Ahluwalia, V.; Walia, S. Plant growth inhibitory terpenes from Eupatorium adenophorum leaves. J. Appl. Bot. Food Qual. 2013, 86, 33–36. [Google Scholar]
- Wang, C.F.; Zhao, Y.; Liu, Y.Z.; Zhang, Z.Z. Occurrence and biological activities of eremophilane-type sesquiterpenes. Chem. Res. Chinese Univ. 2009, 25, 480–484. [Google Scholar]
- He, L.; Hou, J.; Gan, M.; Shi, J.; Chantrapromma, F.S.; Williams, I.D.; Sung, H.H.Y. Cadinane sesquiterpenes from the leaves of Eupatorium adenophorum. J. Nat. Prod. 2008, 71, 1485–1488. [Google Scholar] [CrossRef]
- Moreira, I.C.; Roque, N.F.; Contini, K.; Lago, J.H.G. Sesquiterpenes and hydrocarbons from Xylopia emarginata (Annonaceae) fruits. Rev. Bras. Farmacogn. 2007, 17, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.; Murillo, R.; Castro, V.; Brecht, V.; Merfort, I. Sesquiterpene lactones from Montanoa hibiscifolia that inhibit the transcription factor NF-κB. J. Nat. Prod. 2004, 67, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A. Xanthanolides and xanthane epoxide derivatives from Xanthium strumarium. Planta Med. 1998, 64, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; Sanz, J.F.; Falco, E.; Jakupovic, J.; Lex, J. New oxygenated eudesmanolides from Artemisia herba-alba. Tetrahedron 1990, 46, 7941–7950. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Three new sesquiterpenes from Croton arboreous. J. Nat. Prod. 2004, 67, 914–917. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Faridchehr, A.; Bakhtiyar, M. Sesquiterpene lactones of Iranian Compositae family (Astraceae); their chemical constituents and anti-plasmodial properties of tehranolide (A. Review). Orient J. Chem. 2017, 33. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Taniguchi, H.; Matsukura, Y.; Kawachi, Y.; Shindo, K. Structural elucidation of humulone autoxidation products and analysis of their occurrence in stored hops. J. Nat. Prod. 2014, 77, 1252–1261. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.N.; Fan, C.Q.; Liao, S.G.; Yue, J.M. The first limonoid peroxide in the Meliaceae family: Walsuronoid A from Walsura robusta. Org. Lett. 2007, 9, 2353–2356. [Google Scholar] [CrossRef]
- Chen, G.F.; Li, Z.L.; Tang, C.M.; He, X.; Chen, K.; Pan, D.J.; Hu, C.Q.; McPhail, D.R.; McPhail, A.T.; Lee, K.H. Structure and stereochemistry of pseudolarolide-I, a novel cytotoxic peroxytriterpene dilactone from Pseudolarix kaempferi. Heterocycles 1990, 31, 1903–1906. [Google Scholar]
- Zhou, T.; Zhang, H.; Zhu, N.; Chiu, P. New triterpene peroxides from Pseudolarix kaempferi. Tetrahedron 2004, 60, 4931–4936. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, I.A.; Fronczek, F.R. Revision of the structure of podocarpaside E, from Actaea podocarpa. Acta Crystallograph. 2007, 63, o2101–o2103. [Google Scholar]
- Ding, Y.; Liang, C.; Kim, J.H.; Lee, Y.M.; Hyun, J.H.; Kang, H.K.; Kim, J.A.; Min, B.S.; Kim, Y.H. Triterpene compounds isolated from Acer mandshuricum and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2010, 20, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.P.; Li, N.; Gao, J.; Fu, K.L.; Qin, Y.; Li, G.Y.; Wang, J.H. A new peroxy-multiflorane triterpene ester from the processed seeds of Trichosanthes kirilowii. Helv. Chim. Acta 2011, 94, 1881–1889. [Google Scholar] [CrossRef]
- Chiamg, Y.M.; Kuo, Y.H. New peroxy triterpenes from the aerial roots of Ficus microcarpa. J. Nat. Prod. 2001, 64, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Fei, D.Q.; Chen, S.G.; Gao, K. Antimicrobial triterpenoids from Vladimiria muliensis. J. Nat. Prod. 2008, 71, 547–550. [Google Scholar] [CrossRef]
- Saha, B.; Naskar, D.B.; Misra, D.R.; Pradhan, B.P.; Khastgir, H.N. Baccatin, a novel nor-triterpene peroxide isolated from Sapium baccatum roxb. Tetrahedron Lett. 1977, 26, 3095–3098. [Google Scholar] [CrossRef]
- Wu, Q.X.; Liu, X.; Shi, Y.P. Chemical components from Gentiana aristata. Chem. Biodiver. 2007, 4, 175–179. [Google Scholar] [CrossRef]
- Makino, B.; Kawai, M.; Iwata, Y.; Yamamura, H.; Butsugan, Y.; Ogawa, K.; Hayashi, M. Physalins possessing an endoperoxy structure from Physalis alkekengi var. francheti. Structural revision of physalin K. Bull. Chem. Soc. Japan 1995, 68, 219–223. [Google Scholar] [CrossRef]
- Cirigliano, A.M.; Veleiro, A.S.; Oberti, J.C.; Burton, G. Spiranoid withanolides from Jaborosa odonelliana. J. Nat. Prod. 2002, 65, 1049–1054. [Google Scholar] [CrossRef]
- Casero, C.N.; Oberti, J.C.; Orozco, C.I.; Cárdenas, A.; Brito, I.; Barboza, G.E.; Nicotra, V.E. Withanolides from three species of the genus Deprea (Solanaceae). Chemotaxonomical considerations. Phytochemistry 2015, 110, 83–90. [Google Scholar] [CrossRef]
- Rocha, M.R.; de Souza, J.J.; Barcellos, L.T.; Sant’Anna, C.M.; Braz-Filho, R.; Vieira, I.J. A novel 3,9-(1,2,3-trioxocine)-type steroid of Rauia nodosa (Rutaceae). Molecules 2014, 19, 14637–14648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Pu, J.X.; Huang, S.X.; Wang, Y.Y.; Xiao, W.L.; Li, L.M.; Liu, J.P.; Zhang, H.B.; Li, Y.; Sun, H.D. Schinalactone A, a new cytotoxic triterpenoid from Schisandra sphenanthera. Org. Lett. 2010, 12, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vijver, L.M. Distribution of plumbag in in the Plumbaginaceae. Phytochemistry 1974, 11, 3247–3248. [Google Scholar] [CrossRef]
- Uchiyama, T.; Hara, S.; Makino, M.; Fujimoto, Y. Seco-Adianane-type triterpenoids from Dorstenia brasiliensis (Moraceae). Phytochemistry 2002, 60, 761–764. [Google Scholar] [CrossRef]
- Wautie, A. Prophylaxis and treatment of the chief parasitic diseases of the gastrointestinal tract of the horse. Parasitica 1946, 2, 44–67. [Google Scholar]
- Herz, W.; Watanabe, K.; Kulanthaivel, P.; Blount, J.F. Cycloartanes from Lindheimera texana. Phytochemistry 1985, 24, 2645–2654. [Google Scholar] [CrossRef]
- Dev, S. Handbook of Terpenoids: Triterpenoids, 1st ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume I. [Google Scholar]
- Ageta, H.; Shiojima, K.; Kamaya, R.; Masuda, K. Fern constituent: Naturally occurring adian-5-ene ozonide in the leaves of Adiantum monochlamys and Oleandra wallichii. Tetrahedron Lett. 1978, 19, 899–900. [Google Scholar] [CrossRef]
- Rücker, G.; Manns, D.; Schenkel, E.P.; Hartmann, R.; Heinzmann, B.M. A triterpene ozonide from Senecio Selloi. Arch. Pharm. Med. Chem. 2003, 336, 205–207. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Kazakov, D.V.; Yamansarov, E.Y.; Medvedeva, N.I.; Tolstikov, G.A.; Suponitsky, K.Y.; Arkhipov, D.E. Synthesis of triterpenoid-based 1,2,4-trioxolanes and 1,2,4-dioxazolidines by ozonolysis of allobetulin derivatives. Tetrahedron Lett. 2011, 52, 976–979. [Google Scholar] [CrossRef]
- Omathúna, D.P.; Doskotch, R.W. Amoenolide K and amoenolide K 19-acetate, two grindelane peroxides from Amphiachyris amoena. Isolation, structure determination, and preparation of amoenolide K from amoenolide A by photochemical oxygenation. J. Nat. Prod. 1995, 58, 1407. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Lavaud, C.; Massiot, G.; Skelton, B.W.; Waterman, P.G.; White, A.H. ent-12-Oxolabda-8,13(16)-dien-15-oic acid and ent-8β,12α-epidioxy-12β-hydroxylabda-9(11),13-dien-15-oic acidγ-lactone: Two new diterpenes from the aerial parts of Premna oligotricha. J. Chem. Soc. Perkin Trans. 1991, 1, 893–899. [Google Scholar] [CrossRef]
- Barrero, A.F.; del Moral, J.F.Q.; Aitigri, M. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry 2004, 65, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.; Perez, L.; Rabanal, R.M.; Valverde, S. Diterpenoids from Salvia oxyodon and Salvia lavandulifolia. Phyfochemistry 1983, 22, 585–588. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sanchez, J.F.; Alvarez-Mansaneda, E.J.; Dorado, M.M.; Haidour, A. Endoperoxide diterpenoids and other constituents from Abies marocana. Phytochemistry 1991, 30, 593–597. [Google Scholar] [CrossRef]
- Delgado, G.; Sanchez, E.; Hernandez, J.; Chavez, M.I.; Alvarez, L.; Martinez, E. Abietanoid acid from Lepechinia caulescens. Phytochemistry 1992, 31, 3159–3161. [Google Scholar] [CrossRef]
- San Feliciano, A.; del Corral, J.M.M.; Gordaliza, M.; Castro, M.A. Two diterpenoids from leaves of Juniperus sabina. Phytochemistry 1991, 30, 695–697. [Google Scholar] [CrossRef]
- Maslovskaya, L.A.; Savchenko, A.I.; Pierce, C.J.; Gordon, V.A.; Reddell, P.W.; Parsons, P.G.; Williams, C.M. Unprecedented 1,14-seco-crotofolanes from Croton insularis: Oxidative cleavage of crotofolin C by a putative homo-baeyer-villiger rearrangement. Chem. Eur. J. 2014, 20, 226–14230. [Google Scholar] [CrossRef]
- Maslovskaya, L.A.; Savchenko, A.I.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. Isolation and confirmation of the proposed cleistanthol biogentic link from Croton insularis. Org. Lett. 2011, 13, 1032–1035. [Google Scholar] [CrossRef]
- Qu, J.B.; Zhu, R.L.; Zhang, Y.L.; Guo, H.F.; Wang, X.N.; Xie, C.F.; Yu, W.T.; Ji, M.; Lou, H.X. ent-Kaurane diterpenoids from the liverwort Jungermannia atrobrunnea. J. Nat. Prod. 2008, 71, 1418–1422. [Google Scholar] [CrossRef]
- Guo, F.; Xi, M.; Li, Y. Triptotin A and B, two novel diterpenoids from Tripterygium wilfordii. Tetrahedron Lett. 1999, 40, 947–950. [Google Scholar] [CrossRef]
- Kong, L.Y.; Min, Z.D.; Shi, J.X. Chemical constituents from roots of Jatropha curcas. J. China Pharm. Univ. 1996, 38, 161–166. [Google Scholar]
- Adelekan, A.M.; Prozesky, E.A.; Hussein, A.A.; Urena, L.D.; van Rooyen, P.H.; Liles, D.C.; Meyer, J.J.M.; Rodriguez, B. Bioactive diterpenes and other constituents of Croton steenkampianus. J. Nat. Prod. 2008, 71, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; de Carvalho Meirelles, G.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, X.; Yang, J.; Lao, Y.Z.; Yang, X.W.; Li, X.N.; Zhang, J.J.; Ding, Z.J.; Xu, H.X.; Xu, G. Hypersubones A and B, new polycyclic acylphloroglucinols with intriguing adamantane type cores from Hypericum subsessile. Org. Lett. 2015, 17, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.C.; Chen, C.M.; Zhang, J.W.; Guo, Y.; Tan, D.D.; Wei, G.Z. Hyperisampsins N and O, two new benzoylated phloroglucinol derivatives from Hypericum sampsonii. Chin. Chem. Lett. 2017, 28, 986–990. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Zeng, Y.H.; Mu, Q.; Ka, W.; Shiu, P.; Gibbons, S. Prenylated benzophenone peroxide derivatives from Hypericum sampsonii. Chem. Biodiver. 2010, 7, 953–958. [Google Scholar] [CrossRef]
- Henry, G.E.; Jacobs, H.; Carrington, C.M.S.; McLean, S.; Reynolds, W.F. Prenylated benzophenone derivatives from Caribbean Clusia species (Guttiferae). Plukenetiones B-G and xerophenone A. Tetrahedron 1999, 55, 1581–1596. [Google Scholar] [CrossRef]
- Ishida, Y.; Shirota, O.; Sekita, S. Polyprenylated benzoylphloroglucinoltype derivatives including novel cage compounds from Hypericum erectum. Chem. Pharm. Bull. 2010, 58, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Ting, C.W.; Hwang, T.L.; Chen, I.S.; Cheng, M.J.; Sung, P.J.; Yen, M.H.; Chen, J.J. Garcimultiflorone G, a novel benzoylphloroglucinol derivative from Garcinia multiflora with inhibitory activity on neutrophil pro-inflammatory. Responses. Chem. Biodivers. 2014, 11, 819–824. [Google Scholar] [CrossRef]
- Novais, C.; Kato, L.; Terra, F.; Vaz, M.B.G.; de Oliveira, C.M.A. Goianone, a new natural polyprenylated benzophenone with a homo-adamantyl ketone core spiro-fused to a cyclic peroxide. Planta Med. 2016, 82S, S1–S381. [Google Scholar] [CrossRef]
- Itokawa, H.; Xu, J.; Takeya, K.; Watanabe, K. Studies on chemical constituents of antitumor fraction from Periploca sepium. II.: Structures of new pregnane glycosides, periplocosides A, B and C. Chem. Pharm. Bull. 1988, 36, 982–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, H.; XU, J.; Takeya, K. Studies on chemical constituents of antitumor fraction from Periploca sepium. IV.: Structures of new pregnane glycosides, periplocosides D, E, L, and M. Chem. Pharm. Bull. 1988, 36, 2084–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itokawa, H.; Xu, J.; Takeya, K. Studies on chemical constituents of antitumor fraction from Periploca sepium. V. Structures of new pregnane glycosides, periplocosides J, K, F and O. Chem. Pharm. Bull. 1988, 36, 4441–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez, J.; López, M.; Gibert, E.; Herrero, E.; Luque, F.J. Merging ligand-based and structure-based methods in drug discovery: An overview of combined virtual screening approaches. Molecules 2020, 25, 4723. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Poroikov, V.V. Probabilistic approach in activity prediction. In Chemoinformatics Approaches to Virtual Screening; Varnek, A., Tropsha, A., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 182–216. [Google Scholar]
- Stumpfe, D.; Hu, H.; Bajorath, J. Evolving concept of activity cliffs. ACS Omega 2019, 4, 14360–14368. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G.; Aldous, D.; Raboisson, R.; Rognan, D. (Eds.) The Practice of Medicinal Chemistry, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2015; p. 902. [Google Scholar]
- Mervin, L.H.; Afzal, A.M.; Drakakis, G.; Lewis, R.; Engkvist, O.; Bender, A. Target prediction utilizing negative bioactivity data covering large chemical space. J. Cheminform. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burov, Y.V.; Poroikov, V.V.; Korolchenko, L.V. National system for registration and biological testing of chemical compounds: Facilities for new drugs search. Bull. Natl. Center Biol. Act. Comp. 1990, 1, 4–25. [Google Scholar]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Comp. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Druzhilovskiy, D.S.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Dmitriev, A.V.; Pogodin, P.V.; Poroikov, V.V. Computer-aided prediction of biological activity spectra for chemical compounds: Opportunities and limitations. Biomed. Chem. Res. Meth. 2018, 1, e00004. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.way2drug.com/passonline/ (accessed on 20 October 2020).
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Dmitriev, A.V.; Pogodin, P.V.; Dubovskaya, V.I.; Ivanov, S.M.; Tarasova, O.A.; et al. Computational platform Way2Drug: From the prediction of biological activity to drug repurposing. Russ. Chem. Bull. 2017, 66, 1832–1841. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological and predicted activities of natural azo compounds. Nat. Prod. Bioprospec. 2017, 7, 151–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horishny, V.; Kartsev, V.; Matiychuk, V.; Geronikaki, A.; Anthi, P.; Pogodin, P.; Poroikov, V.; Ivanov, M.; Kostic, M.; Soković, M.D.; et al. 3-Amino-5-(indol-3-yl) methylene-4-oxo-2-thioxothiazolidine derivatives as antimicrobial agents: Synthesis, computational and biological evaluation. Pharmaceuticals 2020, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Amiranashvili, L.; Nadaraia, N.; Merlani, M.; Kamoutsis, C.; Petrou, A.; Geronikaki, A.; Pogodin, P.; Druzhilovskiy, D.; Poroikov, V.; Ciric, A.; et al. Antimicrobial activity of nitrogencontaining 5-alpha-androstane derivatives: In silico and experimental studies. Antibiotics 2020, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological activities of organometalloid (As, At, B, Ge, Si, Se, Te) steroids. J. Appl. Pharm. Sci. 2017, 7, 184–202. [Google Scholar]
- Singh, S.; Bharti, N.; Mohapatra, P.P. Chemistry and biology of synthetic and naturally occurring antiamoebic agents. Chem. Rev. 2009, 109, 1900–1947. [Google Scholar] [CrossRef]
- Teixeira, C.; Vale, N.; Pérez, B.; Gomes, A.; Gomes, J.R.B.; Gomes, P. “Recycling” classical drugs for malaria. Chem. Rev. 2014, 114, 11164–11220. [Google Scholar] [CrossRef] [Green Version]
- Salas, P.F.; Herrmann, C.; Orvig, C. Metalloantimalarials. Chem. Rev. 2013, 5, 3450–3492. [Google Scholar] [CrossRef]
- Vizer, S.A.; Sycheva, E.S.; Quntar, A.A.A.; Kurmankulov, N.B.; Yerzhanov, K.B.; Dembitsky, V.M. Propargylic sulfides: Synthesis, properties, and application. Chem. Rev. 2015, 115, 1475–1502. [Google Scholar] [CrossRef]
- Barnett, D.S.; Guy, R.K. Antimalarials in development in 2014. Chem. Rev. 2014, 114, 11221–11241. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Quntar, A.A.A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 209–237. [Google Scholar] [CrossRef]
- Smoum, R.; Rubinstein, A.; Dembitsky, V.M.; Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012, 112, 4156–4220. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 2006, 41, 883–924. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Levitsky, D.O. Acetylenic terrestrial anticancer agents. Nat. Prod. Commun. 2006, 1, 405–429. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Epoxy acetylenic lipids: Their analogues and derivatives. Prog. Lipid Res. 2014, 56, 67–91. [Google Scholar] [CrossRef]
- Levitsky, D.O.; Dembitsky, V.M. Anti-breast cancer agents derived from plants. Nat. Prod. Bioprosp. 2015, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V. Acetylenic aquatic anticancer agents and related compounds. Nat. Prod. Commun. 2006, 1, 773–811. [Google Scholar] [CrossRef] [Green Version]
- Abu-Lafi, S.; Dembicki, J.W.; Goldshlag, P.; Hanuš, L.O.; Dembitsky, V.M. The use of the Cryogenic GC/MS and on-column injection for study of organosulfur compounds of the Allium sativum. J. Food Composit. Anal. 2004, 17, 235–245. [Google Scholar] [CrossRef]
















| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa ) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 1 | Antiprotozoal (Plasmodium) (0.941) | Antineoplastic (0.929) | Anti-inflammatory (0.963) |
| Apoptosis agonist (0.619) | Antifungal (Candida) (0.638) | ||
| 2 | Antiparasitic (0.812) | Antineoplastic (0.722) | Anti-helmintic (0.761) |
| Antiprotozoal (Plasmodium) (0.735) | Antimetastatic (0.625) | Antifungal (0.702) | |
| 3 | Antiparasitic (0.812) | Antineoplastic (0.722) | Anti-helmintic (0.761) |
| Antiprotozoal (Plasmodium) (0.735) | Antimetastatic (0.625) | Antifungal (0.702) | |
| 4 | Antiparasitic (0.823) | Antineoplastic (0.741) | Anti-helmintic (0.731) |
| Antiprotozoal (Plasmodium) (0.714) | Antimetastatic (0.633) | Antifungal (0.673) | |
| 5 | Antiparasitic (0.806) | Antineoplastic (0.755) | Anti-helmintic (0.832) |
| Antiprotozoal (Plasmodium) (0.742) | Antimetastatic (0.681) | Antifungal (0.689) | |
| 6 | Antiparasitic (0.786) | Antineoplastic (0.722) | Anti-helmintic (0.774) |
| Antiprotozoal (Plasmodium) (0.721) | Antimetastatic (0.625) | Antifungal (0.712) | |
| 7 | Antiparasitic (0.863) | Antineoplastic (0.776) | Anti-helmintic (0.744) |
| Antiprotozoal (Plasmodium) (0.716) | Antimetastatic (0.654) | Antifungal (0.731) | |
| 8 | Antiprotozoal (Plasmodium) (0.922) | Antineoplastic (0.913) | Anti-inflammatory (0.937) |
| 9 | Antiprotozoal (Plasmodium) (0.929) | Antineoplastic (0.929) | Anti-inflammatory (0.929) |
| 10 | Antiprotozoal (Plasmodium) (0.798) | Antineoplastic (0.975) | Alzheimer’s disease treatment (0.745) Neurodegenerative diseases treatment (0.662) |
| 11 | Antiprotozoal (Plasmodium) (0.802) | Antineoplastic (0.983) | Alzheimer’s disease treatment (0.722) |
| 12 | Antiprotozoal (Plasmodium) (0.844) | Antineoplastic (0.921) | Antileukemic (0.599) |
| Chemopreventive (0.703) | Immunosuppressant (0.585) | ||
| 13 | Antiprotozoal (Plasmodium) (0.835) | Antineoplastic (0.912) | Antileukemic (0.602) |
| Chemopreventive (0.658) | Immunosuppressant (0.565) | ||
| 14 | Antiprotozoal (Plasmodium) (0.829) | Antineoplastic (0.915) | Antileukemic (0.599) |
| Chemopreventive (0.644) | Immunosuppressant (0.602) | ||
| 15 | Antiprotozoal (Plasmodium) (0.948) | Antineoplastic (0.835) | Anti-inflammatory (0.576) |
| Antiparasitic (0.542) | Antimetastatic (0.635) | ||
| 16 | Antiprotozoal (Plasmodium) (0.964) | Antineoplastic (0.742) | Antileukemic (0.509) |
| Antiparasitic (0.642) | Antimetastatic (0.518) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 17 | Antiprotozoal (Plasmodium) (0.925) | Apoptosis agonist (0.961) | Atherosclerosis treatment (0.734) |
| Antineoplastic (0.889) | Immunosuppressant (0.721) | ||
| 18 | Antiprotozoal (Plasmodium) (0.889) | Apoptosis agonist (0.867) | Anti-inflammatory (0.815) |
| Antineoplastic (0.841) | Anti-ulcerative (0.736) | ||
| Antimetastatic (0.611) | |||
| 19 | Antiprotozoal (Plasmodium) (0.838) | Apoptosis agonist (0.977) | Atherosclerosis treatment (0.911) |
| Chemopreventive (0.942) | Hypolipemic (0.836) | ||
| Antineoplastic (0.915) | Lipoprotein disorders treatment (0.826) | ||
| Antiparkinsonian, rigidity | Anti-hypercholesterolemic (0.802) | ||
| relieving (0.711) | |||
| Prostate cancer treatment (0.687) | |||
| 20 | Antiprotozoal (Plasmodium) (0.798) | Apoptosis agonist (0.943) | Atherosclerosis treatment (0.738) |
| Antineoplastic (0.767) | Lipoprotein disorders treatment (0.587) | ||
| 21 | Antiprotozoal (Plasmodium) (0.694) | Apoptosis agonist (0.961) | Atherosclerosis treatment (0.628) |
| Chemopreventive (0.733) | Antifungal (0.635) | ||
| Antineoplastic (0.731) | |||
| 22 | Antiprotozoal (Plasmodium) (0.839) | Apoptosis agonist (0.854) | Anti-inflammatory (0.809) |
| Antineoplastic (0.832) | Anti-ulcerative (0.721) | ||
| 23 | Antiprotozoal (Plasmodium) (0.871) | Chemopreventive (0.931) | Atherosclerosis treatment (0.907) |
| Antineoplastic (0.919) | Anti-hypercholesterolemic (0.788) | ||
| 24 | Antiprotozoal (Plasmodium) (0.819) | Apoptosis agonist (0.956) | Atherosclerosis treatment (0.899) |
| Chemopreventive (0.933) | Anti-hypercholesterolemic (0.823) | ||
| Antineoplastic (0.905) | |||
| 25 | Antiprotozoal (Plasmodium) (0.822) | Apoptosis agonist (0.966) | Atherosclerosis treatment (0.919) |
| Antineoplastic (0.929) | Hypolipemic (0.822) | ||
| Prostate cancer treatment (0.699) | Lipoprotein disorders treatment (0.814) | ||
| 26 | Antiprotozoal (Plasmodium) (0.712) | Apoptosis agonist (0.967) | Atherosclerosis treatment (0.635) |
| Antineoplastic (0.740) | Antifungal (0.623) | ||
| 27 | Antiprotozoal (Plasmodium) (0.713) | Apoptosis agonist (0.966) | Atherosclerosis treatment (0.636) |
| Antineoplastic (0.742) | Antifungal (0.624) | ||
| 28 | Antiprotozoal (Plasmodium) (0.776) | Apoptosis agonist (0.929) | Atherosclerosis treatment (0.721) |
| Antineoplastic (0.758) | Lipoprotein disorders treatment (0.632) | ||
| 29 | Antiprotozoal (Plasmodium) (0.778) | Apoptosis agonist (0.922) | Atherosclerosis treatment (0.714) |
| Antineoplastic (0.756) | Lipoprotein disorders treatment (0.599) | ||
| 30 | Antiprotozoal (Plasmodium) (0.711) | Apoptosis agonist (0.971) | Atherosclerosis treatment (0.623) |
| Antineoplastic (0.788) | Antifungal (0.644) | ||
| 31 | Antiprotozoal (Plasmodium) (0.699) | Apoptosis agonist (0.954) | Atherosclerosis treatment (0.699) |
| Antineoplastic (0.737) | Antifungal (0.676) | ||
| 32 | Antiprotozoal (Plasmodium) (0.778) | Apoptosis agonist (0.942) | Atherosclerosis treatment (0.667) |
| Antineoplastic (0.732) | Antifungal (0.645) | ||
| 33 | Antiprotozoal (Plasmodium) (0.778) | Apoptosis agonist (0.961) | Hypolipemic (0.854) |
| Antineoplastic (0.889) | Anti-eczematic (0.812) | ||
| Proliferative diseases treatment (0.522) | Atherosclerosis treatment (0.787) | ||
| 34 | Antiprotozoal (Plasmodium) (0.833) | Apoptosis agonist (0.856) | Anti-inflammatory (0.815) |
| Antineoplastic (0.838) | Anti-ulcerative (0.729) | ||
| 35 | Antiprotozoal (Plasmodium) (0.866) | Apoptosis agonist (0.849) | Anti-inflammatory (0.811) |
| Antineoplastic (0.839) | Antifungal (0.677) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 36 | Antiprotozoal (Plasmodium) (0.884) | Antineoplastic (0.859) | Anti-inflammatory (0.881) |
| Apoptosis agonist (0.719) | Antifungal (0.644) | ||
| 37 | Antiprotozoal (Plasmodium) (0.816) | Antineoplastic (0.725) | Analgesic (0.812) |
| Antiparasitic (0.806) | Antimetastatic (0.625) | Antifungal (0.745) | |
| 38 | Antiprotozoal (Plasmodium) (0.789) | Antineoplastic (0.721) | Analgesic (0.806) |
| Antiparasitic (0.807) | Antimetastatic (0.625) | Antifungal (0.730) | |
| 39 | Antiprotozoal (Plasmodium) (0.765) | Antineoplastic (0.732) | Antileukemic (0.729) |
| Antiparasitic (0.763) | Antimetastatic (0.633) | Antifungal (0.670) | |
| 40 | Antiprotozoal (Plasmodium) (0.778) | Antineoplastic (0.726) | Antileukemic (0.717) |
| Antiparasitic (0.745) | Antimetastatic (0.681) | Antifungal (0.669) | |
| 41 | Antiparasitic (0.814) | Antineoplastic (0.734) | Anti-helmintic (0.765) |
| Antiprotozoal (Plasmodium) (0.744) | Antimetastatic (0.625) | Antifungal (0.708) | |
| 42 | Antiparasitic (0.863) | Antineoplastic (0.799) | Anti-helmintic (0.731) |
| Antiprotozoal (Plasmodium) (0.716) | Antimetastatic (0.689) | Antifungal (0.705) | |
| 43 | Antiprotozoal (Plasmodium) (0.882) | Antineoplastic (0.824) | Anti-inflammatory (0.821) |
| 44 | Antiprotozoal (Plasmodium) (0.702) | Apoptosis agonist (0.910) | Anti-inflammatory (0.686) |
| Antineoplastic (0.782) | Antileukemic (0.659) | ||
| 45 | Antiprotozoal (Plasmodium) (0.898) | Antineoplastic (0.856) | Alzheimer’s disease treatment (0.732) |
| 46 | Antiprotozoal (Plasmodium) (0.775) | Antineoplastic (0.868) | Alzheimer’s disease treatment (0.698) |
| 47 | Antiprotozoal (Plasmodium) (0.844) | Antineoplastic (0.843) | Antifungal (0.659) |
| Chemopreventive (0.712) | Immunosuppressant (0.582) | ||
| 48 | Antiprotozoal (Plasmodium) (0.835) | Antineoplastic (0.823) | Antifungal (0.662) |
| Chemopreventive (0.679) | Immunosuppressant (0.565) | ||
| 49 | Antiprotozoal (Plasmodium) (0.829) | Antineoplastic (0.818) | Antileukemic (0.645) |
| Chemopreventive (0.644) | Immunosuppressant (0.602) | ||
| 50 | Antiprotozoal (Plasmodium) (0.743) | Antineoplastic (0.788) | Antifungal (0.670) |
| Antiparasitic (0.671) | Antimetastatic (0.603) | Anti-inflammatory (0.656) | |
| 51 | Antiprotozoal (Plasmodium) (0.752) | Antineoplastic (0.859) | Antifungal (0.670) |
| Prostate cancer treatment (0.655) | Anti-inflammatory (0.661) | ||
| 52 | Antiprotozoal (Plasmodium) (0.752) | Antineoplastic (0.859) | Analgesic (0.843) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 53 | Antiprotozoal (Plasmodium) (0.911) | Antineoplastic enhancer (0.825) | Antifungal (0.688) |
| Antiparasitic (0.643) | Antineoplastic (0.794) | Anti-inflammatory (0.661) | |
| 54 | Antiprotozoal (Plasmodium) (0.916) | Antineoplastic (0.833) | Antifungal (0.676) |
| Antiparasitic (0.650) | Apoptosis agonist (0.710) | Anti-inflammatory (0.661) | |
| 55 | Antiprotozoal (Plasmodium) (0.919) | Antineoplastic (0.839) | |
| 56 | Antiprotozoal (Plasmodium) (0.904) | Antineoplastic (0.814) | Antifungal (0.646) |
| Antiparasitic (0.649) | Apoptosis agonist (0.670) | Anti-inflammatory (0.589) | |
| 57 | Antiprotozoal (Plasmodium) (0.913) | Antineoplastic (0.795) | Antifungal (0.688) |
| Apoptosis agonist (0.760) | Anti-inflammatory (0.612) | ||
| 58 | Antiprotozoal (Plasmodium) (0.902) | Antineoplastic enhancer (0.825) | Antifungal (0.646) |
| Antiparasitic (0.666) | Antineoplastic (0.794) | Anti-inflammatory (0.611) | |
| 59 | Antiprotozoal (Plasmodium) (0.923) | Antineoplastic (0.865) | Antifungal (0.671) |
| 60 | Antiprotozoal (Plasmodium) (0.908) | Antineoplastic enhancer (0.816) | Antifungal (0.677) |
| Antiparasitic (0.652) | Antineoplastic (0.799) | Anti-inflammatory (0.622) | |
| 61 | Antiprotozoal (Plasmodium) (0.920) | Antineoplastic (0.825) | Antifungal (0.721) |
| 62 | Antiprotozoal (Plasmodium) (0.935) | Antineoplastic (0.716) | Antifungal (0.709) |
| Antineoplastic (renal cancer) (0.598) | |||
| 63 | Antiprotozoal (Plasmodium) (0.839) | Antineoplastic (0.756) | Antifungal (0.705) |
| Antiparasitic (0.780) | Antineoplastic (renal cancer) (0.592) | ||
| 64 | Antiprotozoal (Plasmodium) (0.836) | Antineoplastic (0.758) | Antifungal (0.711) |
| 65 | Antiprotozoal (Plasmodium) (0.835) | Antineoplastic (0.756) | Antifungal (0.705) |
| 66 | Antiprotozoal (Plasmodium) (0.877) | Antineoplastic (0.848) | Antiviral (0.768) |
| 67 | Antiprotozoal (Plasmodium) (0.877) | Antineoplastic (0.848) | Antiviral (0.768) |
| 68 | Antiprotozoal (Plasmodium) (0.938) | Antineoplastic (0.912) | Anti-inflammatory (0.908) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 69 | Antiprotozoal (Plasmodium) (0.930) | Antineoplastic (0.674) | Phobic disorders treatment (0.604) |
| Antimetastatic (0.536) | Ovulation inhibitor (0.550) | ||
| 70 | Antiprotozoal (Plasmodium) (0.756) | Antineoplastic (0.787) | Analgesic (0.883) |
| Antiparasitic (0.662) | Antimetastatic (0.591) | ||
| 71 | Antiprotozoal (Plasmodium) (0.729) | Antineoplastic (0.788) | Analgesic (0.883) |
| Antiparasitic (0.662) | Antimetastatic (0.591) | Antileukemic (0.564) | |
| 72 | Antiprotozoal (Plasmodium) (0.743) | Antineoplastic (0.769) | Analgesic (0.883) |
| Antimetastatic (0.591) | Antileukemic (0.564) | ||
| 73 | Antiprotozoal (Plasmodium) (0.755) | Antineoplastic (0.801) | Analgesic (0.883) |
| Antiparasitic (0.662) | Antimetastatic (0.591) | Antileukemic (0.564) | |
| 74 | Antiprotozoal (Plasmodium) (0.722) | Antineoplastic (0.855) | Analgesic (0.843) |
| Antiparasitic (0.510) | Prostate cancer treatment (0.641) | Anti-inflammatory (0.648) | |
| 75 | Antiprotozoal (Plasmodium) (0.739) | Antineoplastic (0.855) | Analgesic (0.843) |
| Antiparasitic (0.510) | Prostate cancer treatment (0.641) | Antileukemic (0.513) | |
| Antimetastatic (0.517) | Antibacterial (0.503) | ||
| 76 | Antiprotozoal (Plasmodium) (0.964) | Apoptosis agonist (0.862) | Antifungal (0.538) |
| Antineoplastic (0.694) | Antiviral (Arbovirus) (0.536) | ||
| 77 | Antiprotozoal (Plasmodium) (0.954) | Apoptosis agonist (0.910) | Atherosclerosis treatment (0.520) |
| Antiparasitic (0.553) | Antineoplastic (0.768) | ||
| Antimetastatic (0.587) | |||
| 78 | Antiprotozoal (Plasmodium) (0.805) | Antineoplastic (0.949) | Anti-inflammatory (0.924) |
| Apoptosis agonist (0.797) | Antifungal (0.703) | ||
| Antimetastatic (0.505) | |||
| 79 | Antiprotozoal (Plasmodium) (0.855) | Antineoplastic (0.582) | Immunosuppressant (0.706) |
| 80 | Antiprotozoal (Plasmodium) (0.900) | Antineoplastic (0.873) | Anti-psoriatic (0.630) |
| 81 | Antiprotozoal (Plasmodium) (0.964) | Antineoplastic (0.602) | Phobic disorders treatment (0.725) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 82 | Antiprotozoal (Plasmodium) (0.868) | Antineoplastic (0.887) | Anti-inflammatory (0.946) |
| 83 | Antiprotozoal (Plasmodium) (0.809) | Antineoplastic (0.813) | Anti-inflammatory (0.898) |
| 84 | Antiprotozoal (Plasmodium) (0.882) | Apoptosis agonist (0.948) | Anti-inflammatory (0.867) |
| Antineoplastic (0.921) | Antifungal (0.789) | ||
| 85 | Antiprotozoal (Plasmodium) (0.912) | Antineoplastic (0.813) | Cardiotonic (0.939) |
| Cardiovascular analeptic (0.660) | |||
| 86 | Antiprotozoal (Plasmodium) (0.996) | Antineoplastic (0.797) | Antifungal (Candida) (0.915) |
| Antiprotozoal (Toxoplasma) (0.930) | Apoptosis agonist (0.787) | Anti-schistosome (0.911) | |
| Antiprotozoal (Leishmania) (0.923) | DNA synthesis inhibitor (0.747) | Antifungal (Cryptococcus) (0.853) | |
| Antiparasitic (0.869) | Immunosuppressant (0.720) | Diuretic (0.837) | |
| Antiprotozoal (Coccidia) (0.780) | Antifungal (0.827) | ||
| 87 | Antiprotozoal (Plasmodium) (0.996) | Apoptosis agonist (0.919) | Antifungal (Candida) (0.979) |
| Antiprotozoal (Leishmania) (0.966) | Antineoplastic (0.847) | Anti-schistosome (0.961) | |
| Antiprotozoal (Toxoplasma) (0.918) Antiparasitic (0.883) | DNA synthesis inhibitor (0.644) | Antifungal (Cryptococcus) (0.955) | |
| Antiprotozoal (Coccidia) (0.794) | Antifungal (0.846) | ||
| 88 | Antiprotozoal (Plasmodium) (0.996) | Apoptosis agonist (0.919) | Antifungal (Candida) (0.979) |
| Antiprotozoal (Leishmania) (0.966) | Antineoplastic (0.847) | Anti-schistosome (0.961) | |
| Antiprotozoal (Toxoplasma) (0.918) | DNA synthesis inhibitor (0.644) | Antifungal (Cryptococcus) (0.955) | |
| Antiparasitic (0.883) | Antifungal (0.846) | ||
| Antiprotozoal (Coccidia) (0.794) | Angiogenesis inhibitor (0.738) | ||
| 89 | Antiprotozoal (Plasmodium) (0.996) | Apoptosis agonist (0.890) | Antifungal (Candida) (0.976) |
| Antiprotozoal (Leishmania) (0.949) | Antineoplastic (0.820) | Anti-schistosome (0.975) | |
| Antiprotozoal (Toxoplasma) (0.928) | Immunosuppressant (0.704) | Antifungal (Cryptococcus) (0.953) | |
| Antiparasitic (0.880) | DNA synthesis inhibitor (0.590) | Antifungal (0.828) | |
| Antiprotozoal (Coccidia) (0.792) | Antifungal (Aspergillus) (0.627) | ||
| 90 | Antiprotozoal (Plasmodium) (0.996) | Apoptosis agonist (0.866) | Antifungal (Candida) (0.977) |
| Antiprotozoal (Leishmania) (0.957) | Antineoplastic (0.793) | Anti-schistosome (0.970) | |
| Antiprotozoal (Toxoplasma) (0.918) | DNA synthesis inhibitor (0.545) | Antifungal (Cryptococcus) (0.950) | |
| Antiparasitic (0.880) | Antifungal (0.832) | ||
| Antiprotozoal (Coccidia) (0.818) | Antifungal (Aspergillus) (0.761) | ||
| 91 | Antiprotozoal (Plasmodium) (0.982) | Apoptosis agonist (0.787) | Antifungal (Candida) (0.921) |
| Antiprotozoal (Leishmania) (0.966) | Antineoplastic (0.755) | Anti-schistosome (0.915) | |
| Antiparasitic (0.876) | DNA synthesis inhibitor (0.592) | Antifungal (0.849) | |
| Antiprotozoal (Toxoplasma) (0.875) | Antifungal (Cryptococcus) (0.749) | ||
| Antiprotozoal (Coccidia) (0.649) | Antifungal (Aspergillus) (0.631) | ||
| Antiviral (CMV) (0.603) | |||
| 92 | Antiprotozoal (Plasmodium) (0.990) | Apoptosis agonist (0.884) | Anti-schistosome (0.960) |
| Antiprotozoal (Leishmania) (0.929) | Antineoplastic (0.828) | Antifungal (Candida) (0.942) | |
| Antiprotozoal (Toxoplasma) (0.899) | DNA synthesis inhibitor (0.607) | Antifungal (0.868) | |
| Antiparasitic (0.886) | Antifungal (Cryptococcus) (0.825) | ||
| Antiprotozoal (Coccidia) (0.689) | Antiviral (CMV) (0.668) | ||
| Antifungal (Aspergillus) (0.607) | |||
| 93 | Antiprotozoal (Plasmodium) (0.860) | Antineoplastic (0.883) | Anti-eczematic (0.934) |
| Prostate disorders treatment (0.675) | Anti-inflammatory (0.819) | ||
| Cardiovascular analeptic (0.733) | |||
| Anti-psoriatic (0.690) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 94 | Antiprotozoal (Plasmodium) (0.868) | Antineoplastic (0.887) | Anti-inflammatory (0.946) |
| 95 | Antiprotozoal (Plasmodium) (0.874) | Antineoplastic (0.870) | Anti-inflammatory (0.941) |
| 96 | Antiprotozoal (Plasmodium) (0.889) | Antineoplastic (0.769) | Anti-inflammatory (0.918) |
| 97 | Antiprotozoal (Plasmodium) (0.936) | Antineoplastic (0.932) | Anti-inflammatory (0.958) |
| Apoptosis agonist (0.617) | Antifungal (Candida) (0.630) | ||
| 98 | Antiprotozoal (Plasmodium) (0.936) | Antineoplastic (0.932) | Anti-inflammatory (0.958) |
| Apoptosis agonist (0.617) | Antifungal (Candida) (0.630) | ||
| 99 | Antiprotozoal (Plasmodium) (0.928) | Antineoplastic (0.681) | Anti-inflammatory (0.544) |
| 100 | Antiprotozoal (Plasmodium) (0.916) | Antineoplastic (0.854) | Anti-inflammatory (0.945) |
| 101 | Antiprotozoal (Plasmodium) (0.879) | Antineoplastic (0.866) | Anti-inflammatory (0.934) |
| Antiparasitic (0.649) | Antimetastatic (0.623) | Anti-helminthic (0.609) | |
| 102 | Antiprotozoal (Plasmodium) (0.965) | Antineoplastic (0.792) | Carminative (0.652) |
| Antiparasitic (0.576) | Antimetastatic (0.584) | ||
| 103 | Antiprotozoal (Plasmodium) (0.954) | Apoptosis agonist (0.565) | Carminative (0.832) |
| 104 | Antiprotozoal (Plasmodium) (0.956) | Antineoplastic (0.670) | Anti-eczematic (0.700) |
| Antiparasitic (0.574) | Antimetastatic (0.587) | Antifungal (0.593) | |
| 105 | Antiprotozoal (Plasmodium) (0.959) | Antineoplastic (0.678) | Anti-eczematic (0.711) |
| Antiparasitic (0.581) | Antimetastatic (0.588) | Antifungal (0.599) | |
| 106 | Antiprotozoal (Plasmodium) (0.938) | Antineoplastic (sarcoma) (0.734) | Carminative (0.812) |
| 107 | Antiprotozoal (Plasmodium) (0.945) | Antineoplastic (sarcoma) (0.529) | Anti-eczematic (0.715) |
| 108 | Antiprotozoal (Plasmodium) (0.881) | Antineoplastic (0.699) | Anti-eczematic (0.734) |
| 109 | Antiprotozoal (Plasmodium) (0.884) | Antineoplastic (0.862) | Anti-eczematic (0.861) |
| Antiparasitic (0.672) | Apoptosis agonist (0.795) | Anti-inflammatory (0.679) | |
| 110 | Antiprotozoal (Plasmodium) (0.967) | Antineoplastic (0.911) | Anti-eczematic (0.836) |
| Antiparasitic (0.811) | Apoptosis agonist (0.883) | Antifungal (0.812) | |
| Antiprotozoal (Leishmania) (0.731) | DNA synthesis inhibitor (0.652) | Antibacterial (0.667) | |
| 111 | Antiprotozoal (Plasmodium) (0.889) | Antineoplastic (0.769) | Angiogenesis stimulant (0.644) |
| 112 | Antiprotozoal (Plasmodium) (0.917) | Antineoplastic (0.797) | Carminative (0.724) |
| Prostate cancer treatment (0.650) | Anti-inflammatory (0.697) | ||
| 113 | Antiprotozoal (Plasmodium) (0.752) | Antineoplastic (0.946) | Anti-inflammatory (0.949) |
| Apoptosis agonist (0.782) | Anti-eczematic (0.896) | ||
| 114 | Antiprotozoal (Plasmodium) (0.925) | Antineoplastic (0.914) | Anti-eczematic (0.851) |
| Antiparasitic (0.741) | Anti-helmintic (0.702) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 115 | Antiprotozoal (Plasmodium) (0.920) | Antineoplastic (0.719) | Allergic conjunctivitis treatment (0.597) |
| Apoptosis agonist (0.716) | |||
| 116 | Antiprotozoal (Plasmodium) (0.904) | Antineoplastic (0.752) | Anti-inflammatory (0.815) |
| Apoptosis agonist (0.656) | Antifungal (0.533) | ||
| 117 | Antiprotozoal (Plasmodium) (0.886) | Antineoplastic (0.899) | Antifungal (0.807) |
| Antiparasitic (0.548) | Apoptosis agonist (0.852) | Antimitotic (0.690) | |
| 118 | Antiprotozoal (Plasmodium) (0.891) | Antineoplastic (0.902) | Antifungal (0.854) |
| Antiparasitic (0.603) | Apoptosis agonist (0.833) | Antimitotic (0.702) | |
| 119 | Antiprotozoal (Plasmodium) (0.877) | Antineoplastic (0.904) | Antifungal (0.836) |
| Antiparasitic (0.567) | Apoptosis agonist (0.834) | Antimitotic (0.721) | |
| 120 | Antiprotozoal (Plasmodium) (0.878) | Antineoplastic (0.879) | Antifungal (0.823) |
| Antiparasitic (0.601) | Apoptosis agonist (0.821) | Antimitotic (0.704) | |
| 121 | Antiprotozoal (Plasmodium) (0.734) | Antineoplastic (0.828) | Anti-psoriatic (0.607) |
| Chemopreventive (0.785) | Anti-eczematic (0.546) | ||
| 122 | Antiprotozoal (Plasmodium) (0.955) | apoptosis agonist (0.783) | Anti-inflammatory (0.731) |
| Antineoplastic (0.762) | Lipid metabolism regulator (0.617) | ||
| 123 | Antiprotozoal (Plasmodium) (0.702) | Apoptosis agonist (0.910) | Anti-inflammatory (0.686) |
| Antineoplastic (0.782) | Lipid metabolism regulator (0.631) | ||
| 124 | Antiprotozoal (Plasmodium) (0.869) | Apoptosis agonist (0.853) | Anti-inflammatory (0.869) |
| Antiprotozoal (Leishmania) (0.582) | Antineoplastic (0.804) | Antifungal (0.707) | |
| 125 | Antiprotozoal (Plasmodium) (0.848) | Antineoplastic (0.821) | Anti-inflammatory (0.899) |
| 126 | Antiprotozoal (Plasmodium) (0.835) | Apoptosis agonist (0.919) | Anti-inflammatory (0.858) |
| Antineoplastic (0.842) | Diuretic (0.748) | ||
| 127 | Antiprotozoal (Plasmodium) (0.891) | Antineoplastic (0.874) | Hepatoprotectant (0.838) |
| Apoptosis agonist (0.871) | Antifungal (0.716) | ||
| 128 | Antiprotozoal (Plasmodium) (0.891) | Antineoplastic (0.874) | Hepatoprotectant (0.838) |
| Apoptosis agonist (0.871) | Antifungal (0.716) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 129 | Antiprotozoal (0.969) | Antineoplastic (0.812) | Immunosuppressant (0.586) |
| Antiprotozoal (Plasmodium) (0.966) | Antimetastatic (0.504) | Antifungal (0.521) | |
| 130 | Antiprotozoal (0.967) | Antineoplastic (0.819) | Antibacterial (0.657) |
| Antiprotozoal (Plasmodium) (0.966) | Antimetastatic (0.504) | Antifungal (0.521) | |
| 131 | Antiprotozoal (0.837) | Antineoplastic (0.788) | Antifungal (0.609) |
| Antiprotozoal (Plasmodium) (0.820) | Antibacterial (0.557) | ||
| 132 | Antiprotozoal (Plasmodium) (0.883) | Antineoplastic (0.890) | Antifungal (0.666) |
| 133 | Antiprotozoal (Plasmodium) (0.846) | Apoptosis agonist (0.934) | Hypolipemic (0.820) |
| Antineoplastic (0.890) | Anti-hypercholesterolemic (0.608) | ||
| Prostate cancer treatment (0.636) | Atherosclerosis treatment (0.679) | ||
| 134 | Antiprotozoal (Plasmodium) (0.894) | Antineoplastic (0.875) | Antifungal (0.703) |
| 135 | Antiprotozoal (Plasmodium) (0.952) | Antineoplastic (0.756) | Anti-eczematic (0.863) |
| Apoptosis agonist (0.689) | Anti-psoriatic (0.640) | ||
| 136 | Antiprotozoal (Plasmodium) (0.782) | Antineoplastic (0.879) | Anti-eczematic (0.684) |
| Antiprotozoal (0.776) | Antineoplastic (sarcoma) (0.671) | Anti-inflammatory (0.681) | |
| Antineoplastic (renal cancer) (0.615) | |||
| 137 | Antiprotozoal (Plasmodium) (0.908) | Antineoplastic (0.833) | Antifungal (0.714) |
| 138 | Antiprotozoal (Plasmodium) (0.970) | Apoptosis agonist (0.920) | Antifungal (Candida) (0.908) |
| Antiparasitic (0.867) | Antineoplastic (0.850) | Antifungal (Cryptococcus) (0.844) | |
| DNA synthesis inhibitor (0.687) | Antifungal (0.812) | ||
| 139 | Antiprotozoal (Plasmodium) (0.972) | Apoptosis agonist (0.938) | Antifungal (Candida) (0.903) |
| Antiparasitic (0.864) | DNA synthesis inhibitor (0.754) | Antifungal (0.833) | |
| 140 | Antiprotozoal (Plasmodium) (0.977) | Antineoplastic (0.914) | Antifungal (Candida) (0.899) |
| DNA synthesis inhibitor (0.733) | Antifungal (0.834) | ||
| 141 | Antiprotozoal (Plasmodium) (0.894) | Antineoplastic (0.943) | Anti-inflammatory (0.883) |
| 142 | Antiprotozoal (Plasmodium) (0.983) | Apoptosis agonist (0.955) | Antifungal (0.858) |
| Antiparasitic (0.868) | Antineoplastic (0.841) | Antibacterial (0.633) | |
| DNA synthesis inhibitor (0.712) | |||
| 143 | Antiprotozoal (Plasmodium) (0.988) | Apoptosis agonist (0.950) | Antifungal (0.860) |
| Antiparasitic (0.859) | Antineoplastic (0.848) | Antibacterial (0.635) | |
| DNA synthesis inhibitor (0.718) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 144 | Antiprotozoal (Plasmodium) (0.900) | Antineoplastic (0.873) | Respiratory analeptic (0.635) |
| Apoptosis agonist (0.850) | Anti-psoriatic (0.630) | ||
| 145 | Antiprotozoal (Plasmodium) (0.893) | Antineoplastic (0.873) | Respiratory analeptic (0.642) |
| Apoptosis agonist (0.852) | Anti-psoriatic (0.639) | ||
| 146 | Antiprotozoal (Plasmodium) (0.846) | Antineoplastic (0.857) | Antifungal (0.685) |
| 147 | Antiprotozoal (Plasmodium) (0.888) | Antineoplastic (0.824) | Choleretic (0.545) |
| Chemopreventive (0.675) | Immunosuppressant (0.532) | ||
| Apoptosis agonist (0.750) | |||
| 148 | Antiprotozoal (Plasmodium) (0.734) | Antineoplastic (0.798) | |
| Antiparasitic (0.544) | |||
| 149 | Antiprotozoal (Plasmodium) (0.801) | Antineoplastic (0.871) | Prostate disorders treatment (0.627) |
| 150 | Antiprotozoal (Plasmodium) (0.915) | Antineoplastic (0.848) | Antibacterial (0.647) |
| 151 | Antiprotozoal (Plasmodium) (0.772) | Antineoplastic (0.726) | Antileukemic (0.602) |
| 152 | Antiprotozoal (Plasmodium) (0.829) | Antineoplastic (0.685) | Anti-eczematic (0.794) |
| 153 | Antiprotozoal (Plasmodium) (0.905) | Antineoplastic (0.805) | Carminative (0.505) |
| Antiparasitic (0.727) | Antibacterial (0.504) | ||
| 154 | Antiprotozoal (Plasmodium) (0.854) | Antineoplastic (0.804) | Antimitotic (0.597) |
| Antifungal (0.553) |
| No | Antiprotozoal Activity, (Pa) * | Anticancer and Related Activities, (Pa) * | Additional Biological Activities, (Pa) * |
|---|---|---|---|
| 155 | Antiprotozoal (Plasmodium) (0.861) | Antineoplastic (0.788) | Antioxidant (0.551) |
| Antiparasitic (0.503) | Apoptosis agonist (0.784) | Antibacterial (0.505) | |
| Chemopreventive (0.521) | |||
| 156 | Antiprotozoal (Plasmodium) (0.862) | Antineoplastic (0.767) | Hypolipemic (0.581) |
| Apoptosis agonist (0.744) | |||
| 157 | Antiprotozoal (Plasmodium) (0.874) | Antineoplastic (0.761) | Antiviral (Arbovirus) (0.579) |
| Apoptosis agonist (0.743) | |||
| 158 | Antiprotozoal (Plasmodium) (0.875) | Antineoplastic (0.735) | |
| Apoptosis agonist (0.675) | |||
| 159 | Antiprotozoal (Plasmodium) (0.861) | Antineoplastic (0.788) | Antioxidant (0.551) |
| Antiparasitic (0.503) | Apoptosis agonist (0.784) | Antibacterial (0.505) | |
| Chemopreventive (0.521) | |||
| 160 | Antiprotozoal (Plasmodium) (0.875) | Antineoplastic (0.735) | |
| Apoptosis agonist (0.675) | |||
| 161 | Antiprotozoal (Plasmodium) (0.874) | Antineoplastic (0.761) | Antiviral (Arbovirus) (0.579) |
| Apoptosis agonist (0.743) | |||
| 162 | Antiprotozoal (Plasmodium) (0.811) | Apoptosis agonist (0.892) | Antioxidant (0.657) |
| Antineoplastic (0.710) | |||
| 163 | Antiparasitic (0.798) | Antineoplastic (0.958) | Antifungal (0.867) |
| Antiprotozoal (Plasmodium) (0.731) | Apoptosis agonist (0.630) | Antibacterial (0.864) | |
| Antiprotozoal (Leishmania) (0.557) | Cytostatic (0.576) | Immunosuppressant (0.797) | |
| 164 | Antiparasitic (0.779) | Antineoplastic (0.960) | Respiratory analeptic (0.879) |
| Antiprotozoal (Plasmodium) (0.744) | Proliferative diseases treatment (0.740) | Immunosuppressant (0.754) | |
| Chemopreventive (0.666) | Angiogenesis inhibitor (0.569) | ||
| Apoptosis agonist (0.627) | |||
| 165 | Antiparasitic (0.771) | Antineoplastic (0.963) | Respiratory analeptic (0.879) |
| Antiprotozoal (Plasmodium) (0.737) | Proliferative diseases treatment (0.742) | Immunosuppressant (0.754) | |
| Chemopreventive (0.656) | Angiogenesis inhibitor (0.569) | ||
| Apoptosis agonist (0.629) | |||
| 166 | Antiparasitic (0.798) | Antineoplastic (0.959) | Respiratory analeptic (0.936) |
| Antiprotozoal (Plasmodium) (0.732) | Anticarcinogenic (0.732) | Immunosuppressant (0.773) | |
| Chemopreventive (0.731) | Angiogenesis inhibitor (0.620) | ||
| Apoptosis agonist (0.622) | |||
| 167 | Antiparasitic (0.813) | Antineoplastic (0.960) | Immunosuppressant (0.781) |
| Antiprotozoal (Plasmodium) (0.728) | Chemopreventive (0.740) | Anti-inflammatory (0.765) | |
| Apoptosis agonist (0.631) | Analeptic (0.788) | ||
| 168 | Antiparasitic (0.843) | Antineoplastic (0.962) | Respiratory analeptic (0.964) |
| Antiprotozoal (Plasmodium) (0.771) | Proliferative diseases treatment (0.834) | Neuroprotector (0.675) | |
| Apoptosis agonist (0.666) | |||
| Antimetastatic (0.517) | |||
| 169 | Antiprotozoal (Plasmodium) (0.874) | Antineoplastic (0.761) | Antiviral (Arbovirus) (0.579) |
| Apoptosis agonist (0.743) | |||
| 170 | Antiprotozoal (Plasmodium) (0.875) | Antineoplastic (0.735) | |
| Apoptosis agonist (0.675) | |||
| 171 | Antiprotozoal (Plasmodium) (0.861) | Antineoplastic (0.788) | Antioxidant (0.551) |
| Antiparasitic (0.503) | Apoptosis agonist (0.784) | Antibacterial (0.505) | |
| Chemopreventive (0.521) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V. Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules 2021, 26, 686. https://doi.org/10.3390/molecules26030686
Dembitsky VM, Ermolenko E, Savidov N, Gloriozova TA, Poroikov VV. Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules. 2021; 26(3):686. https://doi.org/10.3390/molecules26030686
Chicago/Turabian StyleDembitsky, Valery M., Ekaterina Ermolenko, Nick Savidov, Tatyana A. Gloriozova, and Vladimir V. Poroikov. 2021. "Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity" Molecules 26, no. 3: 686. https://doi.org/10.3390/molecules26030686
APA StyleDembitsky, V. M., Ermolenko, E., Savidov, N., Gloriozova, T. A., & Poroikov, V. V. (2021). Antiprotozoal and Antitumor Activity of Natural Polycyclic Endoperoxides: Origin, Structures and Biological Activity. Molecules, 26(3), 686. https://doi.org/10.3390/molecules26030686