Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection
Abstract
:1. Introduction
2. Research Methodology and Study Selection
- (1)
- Original scientific publications from the year 2010 onwards.
- (2)
- Studies evaluating one or more LFIA test for their ability to detect the following DOA: OPI, BZDs; HRN, THC, MET, AMP, COC, etc.
3. Antibodies Production Processes: Focus on mAbs against Drugs of Abuse
- The molecular size of the injected Ags: the most active immunogens tend to have a high molecular mass (>14,000 Da). Indeed, small Ags (e.g., DOA) are known to be either non-antigenic or weakly antigenic.
- The foreignness: an antigen must be a foreign substance to the animal (not self) to elicit an immune response.
- The chemical complexity: the more complex the immunogen or substance is (chemically), the more immunogenic it will be. The DOA (BZD, heroin, amphetamine, morphine, etc.) are often of low molecular weight and, generally, for any very small Ag, the entire chemical structure is considered by the immune system as a single epitope to which an Ab binds.
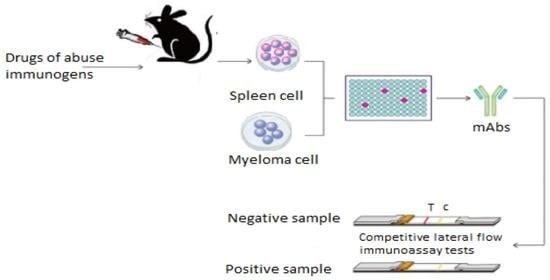
- Step 1: fusing the secretory lymphocyte of an Ab to the Ag used in the animal’s immunization with the myeloma using polyethylene glycol.
- Step 2: identifying the Ab secretory hybridoma.
- Step 3: isolating one cell and maintaining it in culture to obtain a single clone or family of cells, all of which are identical and secretive of the same mAb. It is limit-dilution cloning, and several successive clones are sometimes necessary to obtain a genetically stable clone.
- Step 4: growing the cloned hybridoma in a bioreactor to obtain a mAb concentrate or in a roller system to obtain the less concentrated mAb as a culture supernatant. It can be injected into the abdomen of BALB/c mice (Bagg albino, laboratory-bred strain of the house mouse) to obtain ascites-concentrated mAb.
4. Performances of an Antibody
Applications of mAbs and Their Comparison with Polycolonal Antibodies in the Development of LFIA
5. Lateral Flow Immunoassays (LFIA)
5.1. Basic Components of a LFIA
5.1.1. Sample Pad
5.1.2. Conjugate Pad
- Low non-specific reaction of the sample or the antibody coupled to a nanoparticle (label) (Ab-NP).
- Release of the Ab-NP or the sample should be quick and consistent between individual test strips.
- Ab-NP must remain functional when dried on it.
5.1.3. Test Pad, Reaction Membrane, or Nitrocellulose Membrane
5.1.4. Absorbent Pad or Wick Pad
- The analytes (drugs or proteins, etc.) size and the sample liquid viscosity.
- The porosity and the pore size.
- The thickness (µm) of each membrane.
- The potential coating or treatment that the membrane surface needs.
5.2. Labels
5.3. Formats of LFIA
5.3.1. Sandwich Format

5.3.2. Competitive (or Inhibition) Format

5.3.3. Complex Format or Multiplex Format
5.3.4. The Performance of a LFIA (Validity of the LFIA)
5.3.5. Intrinsic Performances: Sensitivity and Specificity
5.3.6. Extrinsic Performance: Positive (PPV) and Negative Predictive Values (NPV)
5.4. Limitations and Opportunities in LFIA
6. Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of psychotropic drugs on inflammation: Consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Scheifes, A.; Walraven, S.; Stolker, J.J.; Nijman, H.; Egberts, A.C.; Heerdink, E. Adverse events and the relation with quality of life in adults with intellectual disability and challenging behaviour using psychotropic drugs. Res. Dev. Disabil. 2016, 49, 13–21. [Google Scholar] [CrossRef]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnell, K.; Bergman, G.J.; Fastbom, J.; Danielsson, B.; Borg, N.; Salmi, P. Psychotropic drugs and the risk of fall injuries, hospitalisations and mortality among older adults. Int. J. Geriatr. Psychiatry 2016, 32, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shash, D.; Kurth, T.; Bertrand, M.; Dufouil, C.; Barberger-Gateau, P.; Berr, C.; Ritchie, K.; Dartigues, J.-F.; Bégaud, B.; Alpérovitch, A.; et al. Benzodiazepine, psychotropic medication, and dementia: A population-based cohort study. Alzheimer’s Dement. 2016, 12, 604–613. [Google Scholar] [CrossRef]
- Doghramji, K.; Jangro, W.C. Adverse effects of psychotropic medications on sleep. Psychiatr. Clin. N. Am. 2016, 39, 487–502. [Google Scholar] [CrossRef]
- Chang, K.J.; Son, S.J.; Kim, D.; Lee, K.S.; Roh, H.W.; Hong, C.H. P2-111: Effect of psychotropic drugs on development of diabetes mellitus in patients with Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, P526. [Google Scholar] [CrossRef] [Green Version]
- Mawanda, F.; Ms, R.B.W.; Ms, K.M.; Ms, T.E.A. PTSD, Psychotropic medication use, and the risk of dementia among US veterans: A retrospective cohort study. J. Am. Geriatr. Soc. 2017, 65, 1043–1050. [Google Scholar] [CrossRef]
- Sikary, A.K.; Sasidharan, A.; Pillay, V.; Andrade, C. Prescription drug suicide in non-abusers: A 6-year forensic survey. Asian J. Psychiatry 2019, 44, 133–137. [Google Scholar] [CrossRef]
- Qriouet, Z.; Belaiche, A.; Qmichou, Z.; Cherrah, Y.; Sefrioui, H. Benzodiazepines use in Morocco: A nation wide consumption database study between 2004 and 2017. Asian J. Psychiatry 2020, 47, 101852. [Google Scholar] [CrossRef] [PubMed]
- Qriouet, Z.; Qmichou, Z.; Bouchoutrouch, N.; Mahi, H.; Cherrah, Y.; Sefrioui, H. Analytical methods used for the detection and quantification of benzodiazepines. J. Anal. Methods Chem. 2019, 2019, 2035492. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, M.K.; Wiergowski, M.; Aszyk, J.; Kubica, P.; Namieśnik, J.; Biziuk, M. Application of gas chromatography-tandem mass spectrometry for the determination of amphetamine-type stimulants in blood and urine. J. Pharm. Biomed. Anal. 2018, 148, 58–64. [Google Scholar] [CrossRef]
- El-Beqqali, A.; Andersson, L.I.; Jeppsson, A.D.; Abdel-Rehim, M. Molecularly imprinted polymer-sol-gel tablet toward micro-solid phase extraction: II. Determination of amphetamine in human urine samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2017, 1063, 130–135. [Google Scholar] [CrossRef]
- Eckart, K.; Röhrich, J.; Breitmeier, D.; Ferner, M.; Laufenberg-Feldmann, R.; Urban, R. Development of a new multi-analyte assay for the simultaneous detection of opioids in serum and other body fluids using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2015, 1001, 1–8. [Google Scholar] [CrossRef]
- Boumba, V.A.; Rallis, G.; Petrikis, P.; Vougiouklakis, T.; Mavreas, V. Determination of clozapine, and five antidepressants in human plasma, serum and whole blood by gas chromatography–mass spectrometry: A simple tool for clinical and postmortem toxicological analysis. J. Chromatogr. B 2016, 1038, 43–48. [Google Scholar] [CrossRef]
- Pichini, S.; Cortes, L.; Marchei, E.; Solimini, R.; Pacifici, R.; Gómez-Roig, M.D.; García-Algar, O. Ultra-high-pressure liquid chromatography tandem mass spectrometry determination of antidepressant and anxiolytic drugs in neonatal meconium and maternal hair. J. Pharm. Biomed. Anal. 2016, 118, 9–16. [Google Scholar] [CrossRef]
- Gentili, S.; Solimini, R.; Tittarelli, R.; Mannocchi, G.; Busardò, F.P. A Study on the reliability of an on-site oral fluid drug test in a recreational context. J. Anal. Methods Chem. 2016, 2016, 1234581. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cui, D. The application of immunochromatographic analysis in early detection of gastric cancer. In Gastric Cancer Prewarning and Early Diagnosis System; Springer: Berlin/Heidelberg, Germany, 2017; pp. 129–156. [Google Scholar]
- Cao, F.; Xu, J.; Yan, S.; Yuan, X.; Yang, F.; Hou, L.; Zhao, L.; Zeng, L.; Liu, W.; Zhu, L.; et al. A surface plasmon resonance-based inhibition immunoassay for forensic determination of methamphetamine in human serum. Forensic Chem. 2018, 8, 21–27. [Google Scholar] [CrossRef]
- Bailes, J.; Mayoss, S.; Teale, P.; Soloviev, M. Gold nanoparticle antibody conjugates for use in competitive lateral flow assays. In Nanoparticles in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 45–55. [Google Scholar]
- Urusov, A.; Petrakova, A.; Kuzmin, P.; Zherdev, A.; Sveshnikov, P.; Shafeev, G.; Dzantiev, B. Application of gold nanoparticles produced by laser ablation for immunochromatographic assay labeling. Anal. Biochem. 2015, 491, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ion, M.; Moldovan, C.; Dinulescu, S.; Muscalu, G.; Savin, M.; Mihailescu, C.-M.; Stan, D.; Matei, I. Fabrication of a new LFIA test for rapid quantitative detection of CK-MB, using inkjet-printing method. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017. [Google Scholar]
- Clarke, O.J.R.; Goodall, B.L.; Hui, H.P.; Vats, N.; Brosseau, C.L. Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal. Chem. 2017, 89, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Stayton, P.S. Improving lateral-flow immunoassay (LFIA) diagnostics via biomarker enrichment for mHealth. In Mobile Health Technologies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 71–84. [Google Scholar]
- Huang, D.; Ying, H.; Jiang, D.; Liu, F.; Tian, Y.; Du, C.; Zhang, L.; Pu, X. Rapid and sensitive detection of interleukin-6 in serum via time-resolved lateral flow immunoassay. Anal. Biochem. 2020, 588, 113468. [Google Scholar] [CrossRef]
- Cai, Y.; Kang, K.; Liu, Y.; Wang, Y.; He, X. Development of a lateral flow immunoassay of C-reactive protein detection based on red fluorescent nanoparticles. Anal. Biochem. 2018, 556, 129–135. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, T.-H.; Chen, C.-A.; Ho, N.Y.-J.; Chou, Y.-J.; Chen, C.-F. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Sci. Rep. 2018, 8, 17319. [Google Scholar] [CrossRef] [Green Version]
- nanoComposix. Antibody Selection and Purification for Lateral Flow Rapid Tests. 2020. Available online: https://nanocomposix.com/pages/antibody-selection-and-purification-for-lateral-flow-rapid-tests#target (accessed on 2 October 2020).
- Gülpınar, Ö.; Güçlü, A.G. How to write a review article? Turk. J. Urol. 2013, 39 (Suppl. 1), 44. [Google Scholar]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Guo, L.; Liu, L.; Kong, N.; Kuang, H.; Xu, C. Development of an ELISA for nitrazepam based on a monoclonal antibody. Food Agric. Immunol. 2015, 26, 611–621. [Google Scholar] [CrossRef]
- Cuccuru, M.A.; Dessì, D.; Rappelli, P.; Fiori, P.L. A simple, rapid and inexpensive technique to bind small peptides to polystyrene surfaces for immunoenzymatic assays. J. Immunol. Methods 2012, 382, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R. Bovine serum albumin as an immunogenic carrier facilitating the development of hapten-specific monoclonal antibodies. bioRxiv 2020, 1–22. [Google Scholar] [CrossRef]
- Pravetoni, M.; Keyler, D.E.; Pidaparthi, R.; Carroll, F.; Runyon, S.P.; Murtaugh, M.P.; Earley, C.; Pentel, P.R. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem. Pharmacol. 2012, 83, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Liu, L.; Xue, F.; Xing, C.; Song, S.; Kuang, H.; Xu, C. Development of a monoclonal antibody-based immunochromatographic strip for cephalexin. Food Agric. Immunol. 2014, 26, 282–292. [Google Scholar] [CrossRef]
- Cong, X.; Campomanes, P.; Kless, A.; Schapitz, I.; Wagener, M.; Koch, T.; Carloni, P. Structural determinants for the binding of morphinan agonists to the μ-opioid receptor. PLoS ONE 2015, 10, e0135998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapp, R.W., Jr. Clarke’s Analysis of Drugs and Poisons; Moffat, A.C., Osselton, M.D., Widdop, B., Watts, J., Eds.; Pharmaceutical Press: London, UK, 2004; ISBN 0-853-69473-7. [Google Scholar]
- Zola, H. Monoclonal Antibodies: A Manual of Techniques; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Frei, J.; Lai, J.R. Protein and Antibody Engineering by Phage Display. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 45–87. [Google Scholar]
- Teixeira, D.; Gonzalez-Pajuelo, M. Phage Display Technology for Selection of Antibody Fragments. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–88. [Google Scholar]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Koellhoffer, J.F.; Chen, G.; Sandesara, R.G.; Bale, S.; Saphire, E.O.; Chandran, K.; Sidhu, S.S.; Lai, J.R. Two synthetic antibodies that recognize and neutralize distinct proteolytic forms of the ebola virus envelope glycoprotein. ChemBioChem 2012, 13, 2549–2557. [Google Scholar] [CrossRef] [Green Version]
- Dehghannezhad, A.; Paknejad, M.; Rasaee, M.J.; Omidfar, K.; Ebrahimi, S.S.S.; Ghahremani, H. Development of a nanogold-based immunochromatographic assay for detection of morphine in urine using the Amor-HK16 monoclonal antibody. Hybridoma 2012, 31, 411–416. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Ashraf, A.; Ling, S.; Wang, S. In vitro improved production of monoclonal antibody against zearalenone in supplemented cell culture media. PSM Biol. Res. 2018, 3, 106–110. [Google Scholar]
- Silva, B.G.; Tamashiro, W.M.D.S.C.; Ferreira, R.R.; Deffune, E.; Suazo, C.A.T. Assessment of kinetic and metabolic features of two hybridomas in suspension culture for production of two monoclonal antibodies for blood typing. Braz. J. Chem. Eng. 2018, 35, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Rashidian, J.; Copaciu, R.; Su, Q.; Merritt, B.; Johnson, C.; Yahyabeik, A.; French, E.; Cummings, K. Generation and performance of R132H mutant IDH1 rabbit monoclonal antibody. Antibodies 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.L.; Zhang, X.X.; Zhao, Q. Studies on rat-rat hybridoma technique and its application to obtain rat monoclonal antibodies anti-horseradish peroxidase. In Rat Hybridomas and Rat Monoclonal Antibodies (1990); CRC Press: Boca Raton, FL, USA, 2017; pp. 265–270. [Google Scholar]
- Lei, X.; Chen, J.; Yang, P.; Zhao, Y.; Wang, X.; Zhao, J.; Wang, C. The development of the hybridoma cell line secreting monoclonal antibody against S protein of a PEDV variant. Chin. J. Vet. Sci. 2016, 36, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhao, J.; Wang, X.; Zhao, Y.; Wang, C. Development of a hybridoma cell line secreting monoclonal antibody against s protein of a chinese variant of PEDV. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 12–16. [Google Scholar] [CrossRef]
- Alvarado, G.; Crowe, J.E. Development of human monoclonal antibodies against respiratory syncytial virus using a high efficiency human hybridoma technique. In Human Respiratory Syncytial Virus; Springer: Berlin/Heidelberg, Germany, 2016; pp. 63–76. [Google Scholar]
- Morita, I.; Oyama, H.; Yasuo, M.; Matsuda, K.; Katagi, K.; Ito, A.; Tatsuda, H.; Tanaka, H.; Morimoto, S.; Kobayashi, N. Antibody fragments for on-site testing of cannabinoids generated via in vitro affinity maturation. Biol. Pharm. Bull. 2017, 40, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Owens, S.M.; Henry, R.; Brown, A. Anti-(+)—Methamphetamine Monoclonal Antibodies. Worldwide Patent Applications No. CA2901514A1, 14 August 2015. [Google Scholar]
- Ndao, D.; Hickman, D.; López-Deber, M.; Davranche, A.; Pfeifer, A.; Muhs, A. Binding affinity measurement of antibodies from crude hybridoma samples by SPR. BIO-Protocol 2014, 4, e1276. [Google Scholar] [CrossRef]
- Ross, G.M.S.; Bremer, M.G.E.G.; Wichers, J.H.; Van Amerongen, A.; Nielen, M.W.F. Rapid antibody selection using surface plasmon resonance for high-speed and sensitive hazelnut lateral flow prototypes. Biosensors 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozokalfa, G.; Akbulut, H.; Demir, B.; Guler, E.; Gumus, Z.P.; Demirkol, D.O.; Aldemir, E.; Yamada, S.; Endo, T.; Coskunol, H.; et al. Polypeptide functional surface for the aptamer immobilization: Electrochemical cocaine biosensing. Anal. Chem. 2016, 88, 4161–4167. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Heterogeneous and homogeneous immunoassays for drug analysis. Bioanalysis 2014, 6, 2877–2896. [Google Scholar] [CrossRef]
- Wille, S.M.; Di Fazio, V.; Toennes, S.W.; van Wel, J.H.; Ramaekers, J.G.; Samyn, N. Evaluation of Δ9-tetrahydrocannabinol detection using DrugWipe5S® screening and oral fluid quantification after Quantisal™ collection for roadside drug detection via a controlled study with chronic cannabis users. Drug Test. Anal. 2015, 7, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Bertolín, J.R.; Bonel, L.; Asturias, L.; Arcos-Martínez, M.J.; Castillo, J.R. Rapid determination of recent cocaine use with magnetic particles-based enzyme immunoassays in serum, saliva, and urine fluids. J. Pharm. Biomed. Anal. 2016, 125, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, X.Y.; Zhao, W.R. Determination of morphine in human urine by the novel competitive fluorescence immunoassay. J. Anal. Methods Chem. 2019, 2019, 7826090. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-T.; Huang, T.-H.; Ho, N.Y.-J.; Chen, Y.-P.; Chen, C.-A.; Chen, C.-F. Development of a multiplex and sensitive lateral flow immunoassay for the diagnosis of periprosthetic joint infection. Sci. Rep. 2019, 9, 15679. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Microchim. Acta 2019, 186, 621. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, Z.; Pan, Y.; Peng, D.; Wang, J.; Wang, Y.; Yuan, Z. Monoclonal Antibody used for Detecting Benzodiazepine Medicines and Enzyme-Linked Immunosorbent Assay Method and Kit. Patent No. CN104530240A, 22 April 2015. [Google Scholar]
- Hassanpour, S.; Hasanzadeh, M.; Saadati, A.; Shadjou, N.; Soleymani, J.; Jouyban, A. A novel paper based immunoassay of breast cancer specific carbohydrate (CA 15.3) using silver nanoparticles-reduced graphene oxide nano-ink technology: A new platform to construction of microfluidic paper-based analytical devices (μPADs) towards biomedical analysis. Microchem. J. 2019, 146, 345–358. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Xie, Q.-Y.; Wu, Y.-H.; Xiong, Q.-R.; Xu, H.-Y.; Xiong, Y.-H.; Liu, K.; Jin, Y.; Lai, W.-H. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014, 54, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Innova Biosciences. Guide to Lateral Flow Immunoassays; Innova Biosciences: Cambrige, UK, 2017; pp. 1–16. Available online: https://fnkprddata.blob.core.windows.net/domestic/download/pdf/IBS_A_guide_to_lateral_flow_immunoassays.pdf (accessed on 5 February 2021).
- Zhang, X.; Wu, C.; Wen, K.; Jiang, H.; Shena, J.; Zhang, S.; Wanga, Z. Comparison of fluorescent microspheres and colloidal gold as labels in lateral flow immunochromatographic assays for the detection of T-2 toxin. Molecules 2016, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Kang, K.; Li, Q.; Wang, Y.; He, X. Rapid and sensitive detection of cardiac troponin I for point-of-care tests based on red fluorescent microspheres. Molecules 2018, 23, 1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Mao, X.; Juncker, D. Immunochromatographic assay on thread. Anal. Chem. 2012, 84, 7736–7743. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.H. Lateral flow immunoassay. In ELISA; Springer: Berlin/Heidelberg, Germany, 2015; pp. 127–137. [Google Scholar]
- Anfossi, L.; Giovannoli, C.; Giraudi, G.; Biagioli, F.; Passini, C.; Baggiani, C. A lateral flow immunoassay for the rapid detection of Ochratoxin a in wine and grape must. J. Agric. Food Chem. 2012, 60, 11491–11497. [Google Scholar] [CrossRef]
- Clayton, K.N. Comparing Anti-VEGF Antibodies and Aptamers on Paper Microfluidic-based Platforms. Master Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, June 2012. [Google Scholar]
- Gupta, R.; Gupta, S. Lateral Flow Immunoassay for Detecting Vitamins. U.S. Patent Application NO. 14/007,006, 28 December 2014. [Google Scholar]
- Petruno, P.T.; Petrilla, J.F.; Brosnan, M.J.; Zhou, R.; Roitman, D.B. Lateral Flow Assay Systems and Methods. U.S. Patent 8,128,871, 6 March 2012. [Google Scholar]
- Hu, Q.; Wei, Q.; Zhang, P.; Li, S.; Xue, L.; Yang, R.-F.; Wang, C.; Zhou, L. An up-converting phosphor technology-based lateral flow assay for point-of-collection detection of morphine and methamphetamine in saliva. Analyst 2018, 143, 4646–4654. [Google Scholar] [CrossRef]
- Petrakova, A.V.; Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Gold nanoparticles of different shape for bicolor lateral flow test. Anal. Biochem. 2019, 568, 7–13. [Google Scholar] [CrossRef]
- Scherer, J.N.; Fiorentin, T.R.; Borille, B.T.; Pasa, G.; Sousa, T.R.V.; Von Diemen, L.; Limberger, R.P.; Pechansky, F. Reliability of point-of-collection testing devices for drugs of abuse in oral fluid: A systematic review and meta-analysis. J. Pharm. Biomed. Anal. 2017, 143, 77–85. [Google Scholar] [CrossRef]
- Plouffe, B.D.; Murthy, S.K. Fluorescence-based lateral flow assays for rapid oral fluid roadside detection of cannabis use. Electrophoresis 2017, 38, 501–506. [Google Scholar] [CrossRef]
- Market, F.C. Lateral flow immunoassay systems: Evolution from the current state of the art to the next generation of highly sensitive, quantitative rapid assays. Immunoass. Handb. 2013, 89, 89–107. [Google Scholar]
- Bristow, C.C.; Severe, L.; Pape, J.W.; Javanbakht, M.; Lee, S.-J.; Comulada, W.S.; Klausner, J.D. Dual rapid lateral flow immunoassay fingerstick wholeblood testing for syphilis and HIV infections is acceptable and accurate, Port-au-Prince, Haiti. BMC Infect. Dis. 2016, 16, 302. [Google Scholar] [CrossRef] [Green Version]
- Machiesky, L.; Côté, O.; Kirkegaard, L.H.; Mefferd, S.C.; Larkin, C. A rapid lateral flow immunoassay for identity testing of biotherapeutics. J. Immunol. Methods 2019, 474, 112666. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Mirasoli, M.; Anfossi, L.; Nascetti, A.; Caputo, D.; De Cesare, G.; Guardigli, M.; Baggiani, C.; Roda, A. Chemiluminescence lateral flow immunoassay cartridge with integrated amorphous silicon photosensors array for human serum albumin detection in urine samples. Anal. Bioanal. Chem. 2016, 408, 8869–8879. [Google Scholar] [CrossRef] [Green Version]
- Creative Diagnostics. Common Formats of Lateral Flow Tests. 2020. Available online: https://www.cd-diatest.com/common-formats-of-lateral-flow-tests_d27 (accessed on 17 September 2020).
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex lateral flow immunoassay: An overview of strategies towards high-throughput point-of-need testing. Biosensors 2018, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhao, Y.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens. Bioelectron. 2020, 157, 112168. [Google Scholar] [CrossRef] [PubMed]
- Poonlapdecha, W.; Seetang-Nun, Y.; Wonglumsom, W.; Tuitemwong, K.; Erickson, L.E.; Hansen, R.R.; Tuitemwong, P. Antibody-conjugated ferromagnetic nanoparticles with lateral flow test strip assay for rapid detection of Campylobacter jejuni in poultry samples. Int. J. Food Microbiol. 2018, 286, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, T.; Pehrsson, A.; Lillsunde, P.; Vimpari, K.; Houwing, S.; Smink, B.; Mathijssen, R.; Van Der Linden, T.; Legrand, S.-A.; Pil, K.; et al. An analytical evaluation of eight on-site oral fluid drug screening devices using laboratory confirmation results from oral fluid. Forensic Sci. Int. 2011, 208, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Dahanayaka, N.J.; Warnasekara, J.; Rajapakse, R.M.S.R.; Ranathunga, S.Y.K.; Agampodi, S.B. Validity of lateral flow immunochromatographic-assays (LFIA) in diagnosis of leptospirosis. Ceylon Med. J. 2017, 62, 248–249. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Sokullu, E.; Ng, A.; Braescu, L.; Zourob, M. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst 2014, 139, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Karakus, C.; Salih, B.A. Comparison of the lateral flow immunoassays (LFIA) for the diagnosis of Helicobacter pylori infection. J. Immunol. Methods 2013, 396, 8–14. [Google Scholar] [CrossRef]
- Lindsley, M.D.; Mekha, N.; Baggett, H.C.; Surinthong, Y.; Autthateinchai, R.; Sawatwong, P.; Harris, J.R.; Park, B.J.; Chiller, T.; Balajee, S.A.; et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin. Infect. Dis. 2011, 53, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Shan, S.; Lai, W.; Xiong, Y.; Weihua, L.; Xu, H. Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J. Agric. Food Chem. 2015, 63, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.Y.; De Saeger, S.; Liberty, S.; Verheijen, R.; Van Peteghem, C. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal. Bioanal. Chem. 2007, 389, 2103–2107. [Google Scholar] [CrossRef]
- Nara, S.; Tripathi, V.; Singh, H.; Shrivastav, T.G. Colloidal gold probe based rapid immunochromatographic strip assay for cortisol. Anal. Chim. Acta 2010, 682, 66–71. [Google Scholar] [CrossRef]
- Wu, J.; Dong, M.; Zhang, C.; Wang, Y.; Xie, M.; Chen, Y. Magnetic lateral flow strip for the detection of cocaine in urine by naked eyes and smart phone camera. Sensors 2017, 17, 1286. [Google Scholar] [CrossRef] [Green Version]
- Teerinen, T.; Lappalainen, T.; Erho, T. A paper-based lateral flow assay for morphine. Anal. Bioanal. Chem. 2014, 406, 5955–5965. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.J.; Biggs, T.D.; Maughan, M.N.; Feasel, M.G.; Sisco, E.; Sekowski, J.W. Evaluation of a lateral flow immunoassay for the detection of the synthetic opioid fentanyl. Forensic Sci. Int. 2019, 300, 75–81. [Google Scholar] [CrossRef]
- Hudson, M.; Stuchinskaya, T.; Ramma, S.; Patel, J.; Sievers, C.; Goetz, S.; Hines, S.; Menzies, E.; Russell, D.A. Drug screening using the sweat of a fingerprint: Lateral flow detection of Δ9-tetrahydrocannabinol, cocaine, opiates and amphetamine. J. Anal. Toxicol. 2019, 43, 88–95. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Feng, S.; Liu, K.; Wang, P. Development and clinical validation of a sensitive lateral flow assay for rapid urine fentanyl screening in the emergency department. Clin. Chem. 2020, 66, 324–332. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Cao, F.; Zhang, Y.; Lu, J.; Zeng, L. A lateral flow strip based on gold nanoparticles to detect 6-monoacetylmorphine in oral fluid. R. Soc. Open Sci. 2018, 5, 180288. [Google Scholar] [CrossRef]
- Toubou, H.; Namera, A.; Arima, Y.; Uchida, Y.; Torikoshi, A.; Moriya, F.; Nagao, M. Detection of abused drugs in human blood by using the on-site drug-screening device Oratect® III. Leg. Med. 2014, 16, 308–313. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.-N.; Wu, Q.; Yao, L.; Xu, J. Rapid and easy determination of morphine in chafing dish condiments with colloidal gold labeling based lateral flow strips. Food Sci. Hum. Wellness 2019, 8, 40–45. [Google Scholar] [CrossRef]
- Smith, J.E.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. Design and development of aptamer–gold nanoparticle based colorimetric assays for in-the-field applications. JoVE 2016, 112, e54063. [Google Scholar]
- Mousivand, M.; Anfossi, L.; Bagherzadeh, K.; Barbero, N.; Mirzadi-Gohari, A.; Javan-Nikkhah, M. In silico maturation of affinity and selectivity of DNA aptamers against aflatoxin B1 for biosensor development. Anal. Chim. Acta 2020, 1105, 178–186. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. In vitro selection of DNA aptamers targeting β-lactoglobulin and their integration in graphene-based biosensor for the detection of milk allergen. Biosens. Bioelectron. 2017, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, R.; Siaj, M.; Zourob, M. DNA aptamers selection and characterization for development of label-free impedimetric aptasensor for neurotoxin anatoxin-a. Biosens. Bioelectron. 2015, 68, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Abnous, K.; Nabavinia, M.S.; Ramezani, M.; Taghdisi, S.M. In vitro selection of tacrolimus binding aptamer by systematic evolution of ligands by exponential enrichment method for the development of a fluorescent aptasensor for sensitive detection of tacrolimus. J. Pharm. Biomed. Anal. 2020, 177, 112853. [Google Scholar] [CrossRef] [PubMed]
- Zargar, T.; Khayamian, T.; Jafari, M.T. Immobilized aptamer paper spray ionization source for ion mobility spectrometry. J. Pharm. Biomed. Anal. 2017, 132, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafabadi, M.E.; Khayamian, T.; Hashemian, Z. Aptamer-conjugated magnetic nanoparticles for extraction of adenosine from urine followed by electrospray ion mobility spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 244–250. [Google Scholar] [CrossRef]
- Kang, K.; Sachan, A.; Nilsen-Hamilton, M.; Shrotriya, P. Aptamer functionalized microcantilever sensors for cocaine detection. Langmuir 2011, 27, 14696–14702. [Google Scholar] [CrossRef] [Green Version]
- Guler, E.; Bozokalfa, G.; Demir, B.; Gumus, Z.P.; Guler, B.; Aldemir, E.; Timur, S.; Coskunol, H. An aptamer folding-based sensory platform decorated with nanoparticles for simple cocaine testing. Drug Test. Anal. 2016, 9, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shi, Y.; Pan, Y.; Yue, Z.; Zhang, H.; Yi, C. Colorimetric and bare eye determination of urinary methylamphetamine based on the use of aptamers and the salt-induced aggregation of unmodified gold nanoparticles. Microchim. Acta 2014, 182, 505–511. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, M.; Le, X.C.; Li, X.-F. Applications of aptamer affinity chromatography. TrAC Trends Anal. Chem. 2012, 41, 46–57. [Google Scholar] [CrossRef]
- Du, F.; Alam, N.; Pawliszyn, J. Aptamer-functionalized solid phase microextraction–liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal. Chim. Acta 2014, 845, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gan, N.; Cao, Y.; Chen, Y.; Jiang, Q. Selective dispersive solid phase extraction-chromatography tandem mass spectrometry based on aptamer-functionalized UiO-66-NH2 for determination of polychlorinated biphenyls. J. Chromatogr. A 2016, 1446, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lu, D.; Zhang, X.; Chen, Z.; Tan, W. Aptamer-conjugated optical nanomaterials for bioanalysis. TrAC Trends Anal. Chem. 2012, 39, 72–86. [Google Scholar] [CrossRef]
- Song, K.-M.; Cho, M.; Jo, H.; Min, K.; Jeon, S.H.; Kim, T.; Han, M.S.; Ku, J.K.; Ban, C. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal. Biochem. 2011, 415, 175–181. [Google Scholar] [CrossRef]
- Shin, S.; Kim, I.-H.; Kang, W.; Yang, J.K.; Hah, S.S. An alternative to Western blot analysis using RNA aptamer-functionalized quantum dots. Bioorganic Med. Chem. Lett. 2010, 20, 3322–3325. [Google Scholar] [CrossRef]
- Vinkenborg, J.L.; Mayer, G.; Famulok, M. Aptamer-based affinity labeling of proteins. Angew. Chem. Int. Ed. 2012, 51, 9176–9180. [Google Scholar] [CrossRef]
- Cho, S.-J.; Woo, H.-M.; Kim, K.-S.; Oh, J.-W.; Jeong, Y.-J. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011, 112, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhao, Q.; Wang, S.; Zhao, S.; Zhang, S.; Yin, Y.; Dong, Y. Development of a lateral flow aptamer assay strip for facile identification of theranostic exosomes isolated from human lung carcinoma cells. Anal. Biochem. 2020, 594, 113591. [Google Scholar] [CrossRef]
- Dalirirad, S.; Steckl, A.J. Lateral flow assay using aptamer-based sensing for on-site detection of dopamine in urine. Anal. Biochem. 2020, 596, 113637. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-gold nanozyme based competitive lateral flow assay for rapid detection of CA125 in human serum. Biosens. Bioelectron. 2020, 165, 112368. [Google Scholar] [CrossRef]
- Neff, C.P.; Zhou, J.; Remling, L.; Kuruvilla, J.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Rossi, J.J.; Akkina, R. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci. Transl. Med. 2011, 3, 66ra6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Satheesan, S.; Li, H.; Weinberg, M.S.; Morris, K.V.; Burnett, J.C.; Rossi, J.J. Cell-specific RNA aptamer against human CCR5 specifically targets HIV-1 susceptible cells and inhibits HIV-1 infectivity. Chem. Biol. 2015, 22, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Rossi, J.J. Therapeutic potential of aptamer-siRNA conjugates for treatment of HIV-1. BioDrugs 2012, 26, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sefah, K.; Liu, H.; Wang, R.; Tan, W. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc. Natl. Acad. Sci. USA 2009, 107, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Zheng, J.; Song, E.; Donovan, M.; Zhang, K.; Liu, C.; Tan, W. Self-assembled, aptamer-tethered DNA nanotrains for targeted transport of molecular drugs in cancer theranostics. Proc. Natl. Acad. Sci. USA 2013, 110, 7998–8003. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Siddiqui, I.A.; Nihal, M.; Pilla, S.; Rosenthal, K.; Mukhtar, H.; Gong, S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomater. 2013, 34, 5244–5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, M.G.L.; Broomhall, L.S.; du Toit, J.T. Molecular aptamers for drug delivery. Trends Biotechnol. 2011, 29, 634–640. [Google Scholar]
- Zueva, E.; Rubio, L.I.; Ducongé, F.; Tavitian, B. Metastasis-focused cell-based SELEX generates aptamers inhibiting cell migration and invasion. Int. J. Cancer 2010, 128, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Mercier, M.-C.; Dontenwill, M.; Choulier, L. Selection of nucleic acid aptamers targeting tumor cell-surface protein biomarkers. Cancers 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaggiante, B.; Dapas, B.; Farra, R.; Grassi, M.; Pozzato, G.; Giansante, C.; Fiotti, N.; Tamai, E.; Tonon, F.; Grassi, G. Aptamers as targeting delivery devices or anti-cancer drugs for fighting tumors. Curr. Drug Metab. 2013, 14, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Xiao, X.; Li, J.; Li, J.; Yang, H.; Tan, W. Generating lung-metastatic osteosarcoma targeting aptamers for in vivo and clinical tissue imaging. Talanta 2018, 188, 66–73. [Google Scholar] [CrossRef]
- Hu, X.; Tulsieram, K.L.; Zhou, Q.; Mu, L.; Wen, J. Polymeric nanoparticle–aptamer bioconjugates can diminish the toxicity of mercury in vivo. Toxicol. Lett. 2012, 208, 69–74. [Google Scholar] [CrossRef]
- Emrani, A.S.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2016, 79, 288–293. [Google Scholar] [CrossRef]
- Shahdost-Fard, F.; Roushani, M. Conformation switching of an aptamer based on cocaine enhancement on a surface of modified GCE. Talanta 2016, 154, 7–14. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Chen, W.; Liu, L.; Ma, W.; Zhu, Y.; Xu, L.; Kuang, H.; Xu, C. An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosens. Bioelectron. 2011, 26, 3059–3062. [Google Scholar] [CrossRef] [PubMed]





| Test Results | DOA Consumption | Total (n) | |
|---|---|---|---|
| Consumer | Non-Consumer | ||
| Positive (P) | TP | FP | Total P tests |
| Negative (N) | FN | TN | Total N tests |
| Total of consumers | Total of non-consumers | total numbers | |
| Reference | Molecules Detected (Analytes) | LOD (ng/mL) | Confirmatory Methods | Used Sample | Molecules of Revelation (Labels) |
|---|---|---|---|---|---|
| [43] | MOP | 2000 | TLC * | Urine | GNPs |
| [96] | COC | 5 | ELISA ** | MB | |
| [61] | MOP | 0.60 | Not given | MNP | |
| MET | 3 | ||||
| [76] | MOP | 20 | LC-MS *** | Saliva | Up-converting phosphor technology |
| MET | 25 | ||||
| [97] | MOP | 1 | Not given | GNPs | |
| [79] | THC | 0.01 | Fluorescent and Gold Particle | ||
| [57] | THC | 1–10 | UPLC-MS/MS **** | Not given | |
| 1–5 | Serum | ||||
| [98] | OPI | 100 | Not given | Saliva | |
| 8 | Urine | ||||
| [20] | BZDs (Diazepam) | Not given | PBS | GNPs | |
| [96] | COC | 5 | ELISA | Urine | Magnetic bead |
| [99] | THC | 0.19 | LC–MS-MS | Sweat | Not given |
| BZD | 0.09 | ||||
| MOP | 0.068 | ||||
| AMP | 0.08 | ||||
| [100] | Fentanyl (FTY) | 1 | LC-MS/MS | Urine | GNPs |
| [101] | 6-monoacetylmorphine | 4 | UPLC | Saliva | GNPs |
| [102] | MET | 125 | LC-MS | Blood | GNPs |
| AMP | 125 | ||||
| MOP | 50 | ||||
| COD | 50 | ||||
| Dihydrocodeine | 50 | ||||
| Diazepam | 25 | ||||
| Alprazolam | 60 | ||||
| Estazolam | 15 | ||||
| Prazepam | 75 | ||||
| [103] | MOP | 0.1 | Not given | Chafing dish condiments | GNPs |
| References | Molecules Detected | LOD | Used Sample | Confirmatory Methods | Aptamer Used |
|---|---|---|---|---|---|
| [113] | AMP or MET | 0.82 μM | Urine | Not given | 5′-ACG GTT GCA AGT GGG ACT CTG GTA GGC TGG GTT AAT TTG G-3′ |
| [112] | COC | 0.138 nM | Artificial urine | HPLC | 5′-C6-NH2-AGACAAGGAAAATCCTTCAATGAAGTGGGTCGSH2-C3-3′ |
| BE | 1.66 μM | ||||
| [55] | 725.27 ± 3.17 ng/mL | 5′-C6-NH2-AGACAAGGAAAATCCTTCAATGAAGTGGGTCG-SH2-3′ | |||
| 741 ± 1.28 ng/mL | Synthetic Saliva | ||||
| [111] | COC | 5 ± 8.9 μM (1.5 ± 2.7 μg/mL) | Acetonitrile | Not given | 5′-GGGA GAC AAG GAA AAT CCT TCA ATG AAG TGG GTC GACA-3′ 5′-GAC AAG GAA AAT CCT TCA ATG AAG TGG GTC-3′ |
| [137] | 293 pM | Rat serum | Not given | 5′-CCATAGGGAGACAAGGATAAATCCTTCAATGAAGTGGGTCTCCC-Thiol-3′ 5′-FAM ATTGAAGGATTTATCCTT GTCTCCCTATGCTTCAAT-Biotin-3′ | |
| [138] | 5.0 ± 0.1 pmol/L | Human blood serum | Not given | 5′-C6-NH2-AGACAAGG AAAATCCTTCAATGAAGTGGGTCG-SH2-3′ | |
| [139] | OTA | 1 ng/mL | Red wines | ELISA | 5′-GAT CGG GTG TGG GTG GCG TAA AGG GAG CAT CGG ACA AAA AAA AAA AAA AAA AAA-SH-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qriouet, Z.; Cherrah, Y.; Sefrioui, H.; Qmichou, Z. Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules 2021, 26, 1058. https://doi.org/10.3390/molecules26041058
Qriouet Z, Cherrah Y, Sefrioui H, Qmichou Z. Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules. 2021; 26(4):1058. https://doi.org/10.3390/molecules26041058
Chicago/Turabian StyleQriouet, Zidane, Yahia Cherrah, Hassan Sefrioui, and Zineb Qmichou. 2021. "Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection" Molecules 26, no. 4: 1058. https://doi.org/10.3390/molecules26041058
APA StyleQriouet, Z., Cherrah, Y., Sefrioui, H., & Qmichou, Z. (2021). Monoclonal Antibodies Application in Lateral Flow Immunochromatographic Assays for Drugs of Abuse Detection. Molecules, 26(4), 1058. https://doi.org/10.3390/molecules26041058