A Mixed Valence CoIICoIII2 Field-Supported Single Molecule Magnet: Solvent-Dependent Structural Variation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Description of Crystal Structure
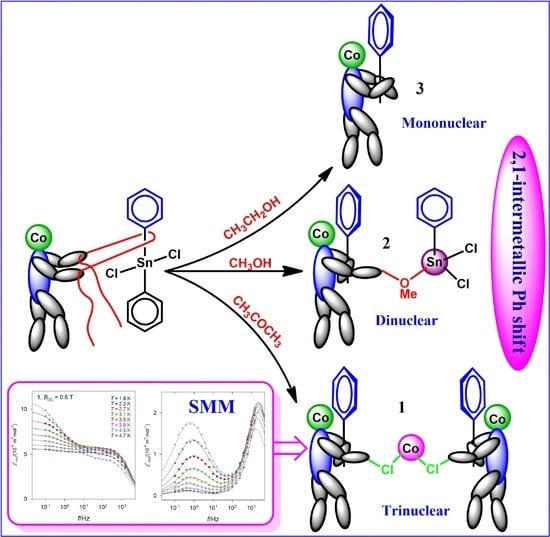
2.3. Solvent Dependent Structural Variation and Rotation of Coordination Geometry
2.4. Magnetic Properties
2.4.1. DC Magnetic Data
2.4.2. Ab Initio Calculations
2.4.3. AC Susceptibility Data
3. Materials and Methods
3.1. Synthesis
3.2. Crystal Structure Determination
3.3. Magnetic Measurements
3.4. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Linert, W.; Verdaguer, M. Molecular Magnets Recent Highlights; Springer-Verlag/Wien 2003: Vienna, Austria, 2003. [Google Scholar]
- Winpenny, R.E.P. Single-Molecule Magnets and Related Phenomena; Springer: Berlin, Germany, 2006. [Google Scholar]
- Rumberger, E.M.W. Magnetization Dynamics in Single-Molecule Magnets. Ph.D. Thesis, University of California, San Diego, CA, USA, 2004. [Google Scholar]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, 1993. [Google Scholar]
- Atanasov, M.; Aravena, D.; Suturina, E.; Bill, E.; Maganas, D.; Neese, F. First principles approach to the electronic structure, magnetic anisotropy and spin relaxation in mononuclear 3d-transition metal single molecule magnets. Coord. Chem. Rev. 2015, 289–290, 177–214. [Google Scholar] [CrossRef]
- Boča, R. Theoretical Foundations of Molecular Magnetism in Current Methods in Inorganic Chemistry; Elsevier: Amsterdam, Netherlands, 1999; pp. 1–874. [Google Scholar]
- Aharoni, A. Introduction to the Theory of Ferromagnetism in International Series of Monographs on Physics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Kirchmayr, H. Magnetic Anisotropy in Encyclopedia of Materials: Science and Technology, 2nd ed.; Pergamon Press: Elsevier, Oxford, UK, 2001; pp. 4754–4757. [Google Scholar]
- Vincent, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Electronic read-out of a single nuclear spin using a molecular spin transistor. Nature 2012, 488, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wernsdorfer, W.; Sessoli, R. Quantum phase interference and parity effects in magnetic molecular clusters. Science 1999, 284, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Cavallini, M.; Gomez-Segura, J.; Ruiz-Molina, D.; Massi, M.; Albonetti, C.; Rovira, C.; Veciana, J.; Biscarini, F. Magnetic information storage on polymers by using patterned single-molecule magnets. Angew. Chem. Int. Ed. 2005, 44, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Heber, J. Information storage. Nature Mater. 2007, 6, 807. [Google Scholar] [CrossRef] [Green Version]
- Tegus, O.; Brück, E.; Buschow, K.H.J.; de Boer, F.R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 2001, 415, 150–152. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Pecharsky, V.K. Thirty years of near room temperature magnetic cooling: Where we are today and future prospects. Int. J. Refrig. 2008, 31, 945–961. [Google Scholar] [CrossRef] [Green Version]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Barra, A.L.; Brunel, L.C.; Guillot, M. Alternating current susceptibility, high field magnetization, and millimeter band EPR evidence for a ground S = 10 state in [Mn12O12(CH3COO)16(H2O)4].2CH3COOH.4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Sessoli, R.; Tsai, H.L.; Schake, A.R.; Wang, S.; Vincent, J.B.; Folting, K.; Gatteschi, D.; Christou, G.; Hendrickson, D.N. High-spin molecules: [Mn12O12(O2CR)16(H2O)4]. J. Am. Chem. Soc. 1993, 115, 1804–1816. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Aubin, S.M.J.; Wemple, M.W.; Adams, D.M.; Tsai, H.-L.; Christou, G.; Hendrickson, D.N. Distorted MnIVMnIII3 cubane complexes as single-molecule magnets. J. Am. Chem. Soc. 1996, 118, 7746–7754. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Perlepes, S.P.; Blatov, V.A.; Proserpio, D.M.; Powell, A.K. High-nuclearity cobalt coordination clusters: Synthetic, topological and magnetic aspects. Coord. Chem. Rev. 2012, 256, 1246–1278. [Google Scholar] [CrossRef] [Green Version]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Christou, G. Azide groups in higher oxidation state manganese cluster chemistry: From structural aesthetics to single-molecule magnets. Inorg. Chem. 2009, 48, 3308–3322. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Abboud, K.A.; Wernsdorfer, W.; Christou, G. Comproportionation reactions to Manganese(III/IV) pivalate clusters: A new half-integer spin single-molecule magnet. Inorg. Chem. 2013, 52, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Boča, R.; Rajnák, C.; Titiš, J.; Valigura, D. Field supported slow magnetic relaxation in a mononuclear Cu(II) complex. Inorg. Chem. 2017, 56, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Cadiou, C.; Murrie, M.; Paulsen, C.; Villar, V.; Wernsdorfer, W.; Winpenny, R.E.P. Studies of a nickel-based single molecule magnet: Resonant quantum tunnelling in an S = 12 molecule. Chem. Commun. 2001, 24, 2666–2667. [Google Scholar] [CrossRef]
- Hazra, S.; Sasmal, S.; Fleck, M.; Grandjean, F.; Sougrati, M.T.; Ghosh, M.; Harris, T.D.; Long, G.J.; Mohanta, S. Slow magnetic relaxation and electron delocalization in an S = 9/2 Iron(II/III) complex with two crystallographically inequivalent iron sites. J. Chem. Phys. 2011, 134, 174507. [Google Scholar] [CrossRef]
- Hoshino, N.; Ako, A.M.; Powell, A.K.; Oshio, H. Molecular magnets containing wheel motifs. Inorg. Chem. 2009, 48, 3396–3407. [Google Scholar] [CrossRef] [PubMed]
- Barra, A.L.; Caneschi, A.; Cornia, A.; de Biani, F.F.; Gatteschi, D.; Sangregorio, C.; Sessoli, R.; Sorace, L. Single-molecule magnet behavior of a tetranuclear Iron(III) complex. The origin of slow magnetic relaxation in Iron(III) clusters. J. Am. Chem. Soc. 1999, 121, 5302–5310. [Google Scholar] [CrossRef]
- Phonsri, W.; Harding, P.; Liu, L.; Telfer, S.G.; Murray, K.S.; Moubaraki, B.; Ross, T.M.; Jameson, G.N.L.; Harding, D.J. Solvent modified spin crossover in an Iron(III) complex: Phase changes and an exceptionally wide hysteresis. Chem. Sci. 2017, 8, 3949–3959. [Google Scholar] [CrossRef] [Green Version]
- Oshio, H.; Hoshino, N.; Ito, T.; Nakano, M. Single-molecule magnets of ferrous cubes: Structurally controlled magnetic anisotropy. J. Am. Chem. Soc. 2004, 126, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- McClain, K.R.; Gould, C.A.; Chakarawet, K.; Teat, S.J.; Groshens, T.J.; Long, J.R.; Harvey, B.J. High-temperature magnetic blocking and magneto-structural correlations in a series of Dysprosium(III) metallocenium single-molecule magnets. Chem. Sci. 2018, 9, 8492–8503. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Lomjanský, D.; Moncoľ, J.; Rajnák, C.; Titiš, J.; Boča, R. Field effects to slow magnetic relaxation in a mononuclear Ni(II) complex. Chem. Commun. 2017, 53, 6930–6932. [Google Scholar] [CrossRef]
- Hazra, S.; Titiš, J.; Valigura, D.; Boca, R.; Mohanta, S. Bis-phenoxido and bis-acetato bridged heteronuclear {CoIIIDyIII} single molecule magnets with two slow relaxation branches. Dalton Trans. 2016, 45, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- Gregson, M.; Chilton, N.F.; Ariciu, A.-M.; Tuna, F.; Crowe, I.F.; Lewis, W.; Blake, A.J.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; et al. A monometallic lanthanide bis(methanediide) single molecule magnet with a large energy barrier and complex spin relaxation behaviour. Chem. Sci. 2016, 7, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Funes, A.V.; Carrella, L.; Rentschler, E.; Alborés, P. {CoIII2DyIII2} single molecule magnet with two resolved thermal activated magnetization relaxation pathways at zero field. Dalton Trans. 2014, 43, 2361–2364. [Google Scholar] [CrossRef]
- Kaemmerer, H.; Baniodeh, A.; Peng, Y.; Moreno-Pineda, E.; Schulze, M.; Anson, C.E.; Wernsdorfer, W.; Schnack, J.; Powell, A.K. Inorganic approach to stabilizing nanoscale toroidicity in a tetraicosanuclear Fe18Dy6 single molecule magnet. J. Am. Chem. Soc. 2020, 142, 14838–14842. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, V.; Hossain, S.; Das, S.; Biswas, S.; Sutter, J.-P. Rhombus-shaped tetranuclear [Ln4] complexes [Ln = Dy(III) and Ho(III)]: Synthesis, structure, and SMM behavior. Inorg. Chem. 2013, 52, 6346–6353. [Google Scholar] [CrossRef] [PubMed]
- Boča, R.; Miklovic, J.; Titiš, J. Simple mononuclear Cobalt(II) complex: A single-molecule magnet showing two slow relaxation processes. Inorg. Chem. 2014, 53, 2367–2369. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, R.; Pedersen, K.S.; Ueda, T.; Suzuki, T.; Bendix, J.; Mikuriya, M. Field-induced single-molecule magnet behavior in ideal trigonal antiprismatic Cobalt(II) complexes: Precise geometrical control by a hydrogen-bonded rigid metalloligand. Chem. Commun. 2018, 54, 8869–8872. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Long, J.R. Slow magnetic relaxation at zero field in the tetrahedral complex [Co(SPh)4]2–. J. Am. Chem. Soc. 2011, 133, 20732–20734. [Google Scholar] [CrossRef] [PubMed]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, Y.O.; Skelton, B.W.; Ozarowski, A.; Titiš, J.; Vranovičová, B.; Boča, R. Field-assisted slow magnetic relaxation in a six-coordinate Co(II)–Co(III) complex with large negative anisotropy. Inorg. Chem. 2017, 56, 6999–7009. [Google Scholar] [CrossRef]
- Ferguson, A.; Parkin, A.; Sanchez-Benitez, J.; Kamenev, K.; Wernsdorfer, W.; Murrie, M. A mixed-valence Co7 single-molecule magnet with C3 symmetry. Chem. Commun. 2007, 3473–3475. [Google Scholar] [CrossRef]
- Rajnák, C.; Varga, F.; Titiš, J.; Moncoľ, J.; Boča, R. Octahedral–tetrahedral systems [Co(dppmO,O)3]2+[CoX4]2– showing slow magnetic relaxation with two relaxation modes. Inorg. Chem. 2018, 57, 4352–4358. [Google Scholar] [CrossRef]
- Varga, F.; Rajnák, C.; Titiš, J.; Moncoľ, J.; Boča, R. Slow magnetic relaxation in a Co(II) octahedral–tetrahedral system formed of a [CoL3]2+ core with L = bis(diphenylphosphanoxido) methane and tetrahedral [CoBr4]2− counter anions. Dalton Trans. 2017, 46, 4148–4151. [Google Scholar] [CrossRef]
- Valigura, D.; Rajnák, C.; Moncoľ, J.; Titiš, J.; Boča, R. A mononuclear Co(II) complex formed from pyridinedimethanol with manifold slow relaxation channels. Dalton Trans. 2017, 46, 10950–10956. [Google Scholar] [CrossRef]
- Rajnák, C.; Dlháň, Ľ.; Moncol, J.; Titiš, J.; Boča, R. Slow magnetic relaxation in a μ1,1′-azido cobalt(II) methylquinoline chain complex. Dalton Trans. 2018, 47, 15745–15750. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Cui, C.; Zhang, Y.-Q.; Jia, J.-H.; Guo, X.; Gao, C.; Qian, K.; Jiang, S.-D.; Wang, B.-W.; Wang, Z.-M.; et al. Zero-field slow magnetic relaxation from single Co(II) ion: A transition metal single-molecule magnet with high anisotropy barrier. Chem. Sci. 2013, 4, 1802–1806. [Google Scholar] [CrossRef]
- Hazra, S.; Mohanta, S. Metal–tin derivatives of compartmental Schiff Bases: Synthesis, structure and application. Coord. Chem. Rev. 2019, 395, 1–24. [Google Scholar] [CrossRef]
- Hazra, S.; Chakraborty, P.; Mohanta, S. Heterometallic Copper(II)–Tin(II/IV) salts, cocrystals, and salt cocrystals: Selectivity and structural diversity depending on ligand substitution and the metal oxidation state. Cryst. Growth Des. 2016, 16, 3777–3790. [Google Scholar] [CrossRef]
- Hazra, S.; Meyrelles, R.; Charmier, A.J.; Rijo, P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. N–H⋯O and N–H⋯Cl supported 1D chains of heterobimetallic CuII/NiII–SnIV cocrystals. Dalton Trans. 2016, 45, 17929–17938. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Martins, N.M.R.; Kuznetsov, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Flexibility and lability of a phenyl ligand in hetero-organometallic 3d metal–Sn(IV) compounds and their catalytic activity in Baeyer–Villiger oxidation of cyclohexanone. Dalton Trans. 2017, 46, 13364–13375. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. Tricyclische orthokondensierte Nebenvalenzringe. Justus Liebigs Ann. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Tang, J.; Huang, F.; Wei, Y.; Bian, H.; Zhang, W.; Liang, H. Bovine serum albumin–Cobalt(II) Schiff base complex hybrid: An efficient artificial metalloenzyme for enantioselective sulfoxidation using hydrogen peroxide. Dalton Trans. 2016, 45, 8061–8072. [Google Scholar] [CrossRef]
- Mechi, L.; Siega, P.; Dreos, R.; Zangrando, E.; Randaccio, L. Crystal structures and solution behavior of paramagnetic, trinuclear, mixed-valent cobalt complexes with Salen-type ligands. Eur. J. Inorg. Chem. 2009, 2629–2638. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Jarzembska, K.N.; Kamiński, R.; Durka, K.; Kubsik, M. Engineering of solvatomorphs of the luminescent complex of ortho-phenylenediboronic acid and 8-hydroxyquinoline. Cryst. Growth Des. 2017, 17, 6836–6851. [Google Scholar] [CrossRef]
- Hazra, S.; Guedes da Silva, M.F.C.; Karmakar, A.; Pombeiro, A.J.L. 1D hacksaw chain bipyridine–sulfonate Schiff base-dicopper(II) as a host for variable solvent guests. RSC Adv. 2015, 5, 28070–28079. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Dey, A.; Mota, A.J.; Colacio, E. Slow magnetic relaxation in Co(III)–Co(II) mixed-valence dinuclear complexes with a CoIIO5X (X = Cl, Br, NO3) distorted-octahedral coordination sphere. Inorg. Chem. 2013, 52, 4554–4561. [Google Scholar] [CrossRef]
- Shiga, T.; Onuki, T.; Matsumoto, T.; Nojiri, H.; Newton, G.N.; Hoshino, N.; Oshio, H. Undecanuclear mixed-valence 3d–4f bimetallic clusters. Chem. Commun. 2009, 3568–3570. [Google Scholar] [CrossRef] [PubMed]
- Brechin, E.K.; Huffman, J.C.; Christou, G.; Yoo, J.; Nakano, M.; Hendrickson, D.N. A new class of single-molecule magnets: Mixed-valent [Mn4(O2CMe)2(Hpdm)6][ClO4]2 with an S = 8 ground state. Chem. Commun. 1999, 783–784. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Yao, S.; Li, Y.-G.; Wu, H.-H.; Wang, Y.-H.; Rouzières, M.; Clérac, R.; Su, Z.-M.; Wang, E.-B. A polyoxometalate-based single-molecule magnet with a mixed-valent {MnIV2MnIII6MnII4} core. Chem. Commun. 2013, 49, 2515–2517. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Yamaguchi, A.; Nakano, M.; Krzystek, J.; Streib, W.E.; Brunel, L.-C.; Ishimoto, H.; Christou, G.; Hendrickson, D.N. Mixed-valence tetranuclear manganese single-molecule magnets. Inorg. Chem. 2001, 40, 4604–4616. [Google Scholar] [CrossRef] [Green Version]
- Stamatatos, T.C.; Nastopoulos, V.; Tasiopoulos, A.J.; Moushi, E.E.; Wernsdorfer, W.; Christou, G.; Perlepes, S.P. High nuclearity single-molecule magnets: A mixed-valence Mn26 cluster containing the Di-2-pyridylketone diolate dianion. Inorg. Chem. 2008, 47, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Ako, A.M.; Mereacre, V.; Hewitt, I.J.; Clérac, R.; Lecren, L.; Anson, C.E.; Powell, A.K. Enhancing single molecule magnet parameters. Synthesis, crystal structures and magnetic properties of mixed-valent Mn4 SMMs. J. Mater. Chem. 2006, 16, 2579–2586. [Google Scholar] [CrossRef]
- Boča, R. A Handbook of Magnetochemical Formulae; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- APEX2, SMART, SAINT & SADABS: Bruker; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, 71, 9–18. [Google Scholar]
- Neese, F. The ORCA program system, WIREs. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. ORCA—An Ab Initio, Density Functional and Semi-empirical Program Package, Version 4.0.0; phpBB: Ruhr, Germany, 2017. [Google Scholar]
- Atanasov, M.; Ganyushin, D.; Pantazis, D.A.; Sivalingam, K.; Neese, F. Detailed ab initio first-principles study of the magnetic anisotropy in a family of trigonal pyramidal Iron(II) pyrrolide complexes. Inorg. Chem. 2011, 50, 7460–7477. [Google Scholar] [CrossRef] [Green Version]
- Angeli, C.; Borini, S.; Cestari, M.; Cimiraglia, R. A quasidegenerate formulation of the second order n-electron valence state perturbation theory approach. J. Chem. Phys. 2004, 121, 4043. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef]
- Ganyushin, D.; Neese, F. First-principles calculations of zero-field splitting parameters. J. Chem. Phys. 2006, 125, 024103. [Google Scholar] [CrossRef]
- Neese, F. Calculation of the zero-field splitting tensor on the basis of hybrid density functional and Hartree-Fock theory. J. Chem. Phys. 2007, 127, 164112. [Google Scholar] [CrossRef] [PubMed]










| 1 (at 150 K) | 1 (at 298 K) | XUHSUP | XUHTAW | XUHTEA | |
|---|---|---|---|---|---|
| CoIICoIII2 | CoIICoIII2 | CoIICoIII2 | CoIICoIII2 | CoIICoIII2 | |
| CCDC | 2016605 | 2016606 | 717829 | 717830 | 717831 |
| CoIII–C | 1.934(2) 1.936(2) | 1.930(5) 1.937(4) | 1.939(9) 1.948(9) 1.951(1) 2.077(1) | 1.965(7) | 1.952(7) 2.074(10) |
| CoIII–N | 1.862(2) 1.864(2) 1.865(2) 1.871(2) | 1.850(4) 1.859(4) 1.863(4) 1.868(3) | 1.844(8) 1.902(7) 1.879(7) 1.891(8) 1.880(7) 1.895(6) 1.844(8) 1.868(8) | 1.879(5) 1.886(4) | 1.861(6) 1.864(5) 1.881(5) 1.884(5) |
| CoIII–O | 1.894(2) 1.896(2) 1.903(2) 1.904(2) | 1.894(3) 1.895(3) 1.900(3) 1.906(3) | 1.864(5) 1.871(6) 1.872(6) 1.876(5) 1.878(5) 1.889(6) 1.892(6) 1.896(6) | 1.875(4) 1.881(3) | 1.866(4) 1.879(4) 1.879(4) 1.887(5) |
| CoIII–Cl | 2.565(6) 2.636(7) | 2.573(1) 2.657(1) | 2.507(3) 2.532(3) 2.604(3) 2.625(3) | 2.561(2) | 2.599(2) 2.516(2) |
| CoII–O | 2.092(2) 2.114(2) 2.124(2) 2.125(2) | 2.092(3) 2.121(3) 2.125(3) 2.138(3) | 2.080(6) 2.081(5) 2.083(6) 2.088(6) 2.100(6) 2.136(6) 2.194(6) 2.202(6) | 2.091(4) 2.094(4) | 2.097(4) 2.104(4) 2.112(4) 2.113(4) |
| CoII–Cl | 2.401(7) 2.409(7) | 2.395(1) 2.409(1) | 2.408(2) 2.409(3) 2.414(3) 2.425(3) | 2.441(2) | 2.402(2) 2.407(2) |
| CoIII···CoII | 2.835(1) 2.849(1) | 2.848(1) 2.859(1) | 2.839(2) 2.843(2) 2.850(2) 2.854(2) | 2.832(1) | 2.835(1) 2.836(1) |
| N–CoIII–O (trans) | 171.25(8) 171.86(8) 172.94(8) 174.67(8) | 171.16(1) 171.99(1) 172.80(1) 174.62(1) | 171.3(3) 172.3(3) 172.3(3) 172.5(3) 172.9(3) 174.6(3) 174.8(3) 176.7(3) | 172.77(19) 172.77(18) | 171.5(2) 174.2(2) 175.0(2) 172.3(2) |
| O–CoII–O (trans) | 172.23(6) | 171.95(1) | 174.9(2) 177.2(2) | 180.0 180.0 | 176.3(2) |
| O–CoII–Cl (trans) | 170.20(5) 169.57(5) | 169.93(8) 170.79(9) | 164.48(16) 164.86(18) 166.95(18) 167.43(17) | - | 167.10(13) 167.98(12) |
| Cl–CoII–Cl | 105.27(2) | 104.80(4) | 105.35(9) 109.28(9) | 180.0 | 105.79(6) |
| CoIII–O–CoII | 89.32(6) 90.09(6) 90.17(6) 90.51(6) | 89.74(1) 90.12(1) 90.46(1) 91.05(1) | 88.2(2) 91.3(2) 90.2(2) 91.7(2) 88.4(2) 92.2(2) 91.2(2) 91.0(2) | 90.79(14) 90.87(15) | 90.33(16) 90.34(16) 90.58(17) 90.94(16) |
| CoIII–Cl–CoII | 68.712(18) 69.425(18) | 68.71(3) 69.63(3) | 68.43(7) 70.21(7) 69.17(7) 70.70(7) | 68.91(5) | 68.86(5) 70.38(5) |
| CoIII···CoII···CoIII | 158.68 | 157.94 | 163.50(6) 163.70(6) | 180.0 | 163.51(4) |
| 1. Term energies: quartet-to-quartet transition energies/cm−1 | {0, 394, 1318}(4T1g), {7061, 7365, 7630}(4T2g), 14988(4A2g), {20620, 20951, 21786}(4T1g) |
| 2. Effect of SOI: energies of spin-orbit multiplets (Kramers doublets) of the ground term/cm−1 | 0, 195, 645, 952, 1653, 1763 |
| 3. Spin Hamiltonian parameters | |
| g1,2,3; gav | 1.904, 2.303, 3.012; 2.41 |
| D/hc/cm−1 | −85.27 |
| E/D | 0.32 |
| BDC/T | R(χ′)/% | R(χ″)/% | χS | χLF | αLF | τLF/s | χHF | αHF | τHF/10−6 s | xLF |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.48 | 1.4 | 2.3(2) | 4.8(3) | 0.33(2) | 0.08(1) | 13.6(1) | 0.07(1) | 93(4) | 0.22 |
| 0.4 | 0.49 | 2.4 | 0.63(18) | 6.4(2) | 0.38(1) | 0.15(1) | 12.7(1) | 0.13(2) | 107(5) | 0.48 |
| 0.6 | 0.87 | 1.9 | 0.59(25) | 7.4(3) | 0.39(1) | 0.25(1) | 11.1(1) | 0.11(3) | 87(8) | 0.64 |
| 0.8 | 0.79 | 2.4 | 0.02 | 7.1(8) | 0.43(1) | 0.44(2) | 9.91(10) | 0.14(6) | 36(14) | 0.72 |
| 1.0 | 0.93 | 2.5 | 0.08 | 6.2(17) | 0.44(1) | 0.59(3) | 8.13(10) | 0.20(13) | 17.3 | 0.76 |
| T/K | R(χ′)/% | R(χ″)/% | χS | χLF | αLF | τLF/s | χHF | αHF | τHF/10−6 s | xLF |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.9 | 0.84 | 1.9 | 0.32(29) | 7.1(3) | 0.38(1) | 0.25(1) | 11.1(1) | 0.14(3) | 78(9) | 0.63 |
| 2.3 | 0.92 | 2.9 | 0.44(34) | 5.5(4) | 0.37(1) | 0.19(1) | 9.9(1) | 0.12(3) | 76(9) | 0.53 |
| 2.7 | 0.49 | 1.9 | 0.40(20) | 3.9(2) | 0.36(1) | 0.16(1) | 8.8(1) | 0.13(1) | 73(5) | 0.41 |
| 3.1 | 0.49 | 2.2 | 0.41(20) | 2.7(2) | 0.34(1) | 0.17(1) | 8.0(1) | 0.13(2) | 71(4) | 0.31 |
| 3.5 | 0.53 | 2.8 | 0.40(22) | 1.9(2) | 0.33(2) | 0.22(2) | 7.2(1) | 0.10(2) | 69(4) | 0.22 |
| 3.9 | 0.52 | 3.7 | 0.50(24) | 1.5(3) | 0.34(4) | 0.28(4) | 6.6(1) | 0.09(2) | 66(4) | 0.17 |
| 4.3 | 0.44 | 2.3 | 0.46(19) | 1.1(2) | 0.30(4) | 0.30(4) | 6.1(1) | 0.08(1) | 60(3) | 0.11 |
| 4.7 | 0.41 | 1.8 | 0.61(18) | 1.0(2) | 0.33(6) | 0.37(7) | 5.6(1) | 0.06(1) | 55(3) | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazra, S.; Rajnák, C.; Titiš, J.; Guedes da Silva, M.F.C.; Boča, R.; Pombeiro, A.J.L. A Mixed Valence CoIICoIII2 Field-Supported Single Molecule Magnet: Solvent-Dependent Structural Variation. Molecules 2021, 26, 1060. https://doi.org/10.3390/molecules26041060
Hazra S, Rajnák C, Titiš J, Guedes da Silva MFC, Boča R, Pombeiro AJL. A Mixed Valence CoIICoIII2 Field-Supported Single Molecule Magnet: Solvent-Dependent Structural Variation. Molecules. 2021; 26(4):1060. https://doi.org/10.3390/molecules26041060
Chicago/Turabian StyleHazra, Susanta, Cyril Rajnák, Ján Titiš, M. Fátima C. Guedes da Silva, Roman Boča, and Armando J. L. Pombeiro. 2021. "A Mixed Valence CoIICoIII2 Field-Supported Single Molecule Magnet: Solvent-Dependent Structural Variation" Molecules 26, no. 4: 1060. https://doi.org/10.3390/molecules26041060
APA StyleHazra, S., Rajnák, C., Titiš, J., Guedes da Silva, M. F. C., Boča, R., & Pombeiro, A. J. L. (2021). A Mixed Valence CoIICoIII2 Field-Supported Single Molecule Magnet: Solvent-Dependent Structural Variation. Molecules, 26(4), 1060. https://doi.org/10.3390/molecules26041060