Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Route of Probe 1
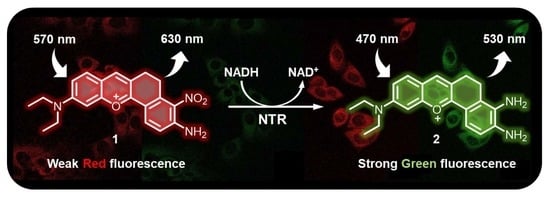
2.2. Sensing Mechanism of Probe 1
2.3. Photophysical Properties of Probe 1
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. Biological Applications of Probe 1 Using Confocal Microscopy
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Cell Culture
3.3. Synthesis of Probe 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Parkinson, G.N.; Skelly, J.V.; Neidle, S. Crystal structure of FMN-dependent nitroreductase from Escherichia coli B: A prodrug-activating enzyme. J. Med. Chem. 2000, 43, 3624–3631. [Google Scholar] [CrossRef]
- Lovering, A.L.; Hyde, E.I.; Searle, P.F.; White, S.A. The structure of Escherichia coli nitroreductase complexed with nicotinic acid: Three crystal forms at 1.7 Å, 1.8 Å and 2.4 Å resolution. J. Mol. Biol. 2001, 309, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.D.; Pérez-Reinado, E.; Castillo, F.; Moreno-Vivián, C. Reduction of polynitroaromatic compounds: The bacterial nitroreductases. FEMS Microbiol. Rev. 2008, 32, 474–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klockow, J.L.; Hettie, K.S.; LaGory, E.L.; Moon, E.J.; Giaccia, A.J.; Graves, E.E.; Chin, F.T. An activatable NIR fluorescent rosol for selectively imaging nitroreductase activity. Sens. Actuators B Chem. 2020, 306, 127446. [Google Scholar] [CrossRef]
- Searle, P.F.; Chen, M.J.; Hu, L.; Race, P.R.; Lovering, A.L.; Grove, J.I.; Guise, C.; Jaberipour, M.; James, N.D.; Mautner, V.; et al. Nitroreductase: A prodrug-activating enzyme for cancer gene therapy. Clin. Exp. Pharmacol. Physiol. 2004, 31, 811–816. [Google Scholar] [CrossRef]
- Liu, Z.; Song, F.; Shi, W.; Gurzadyan, G.; Yin, H.; Song, B.; Liang, R.; Peng, X. Nitroreductase-Activatable Theranostic Molecules with High PDT Efficiency under Mild Hypoxia Based on a TADF Fluorescein Derivative. ACS Appl. Mater. Interfaces 2019, 11, 15426–15435. [Google Scholar] [CrossRef]
- Pacheco-Torres, J.; López-Larrubia, P.; Ballesteros, P.; Cerdán, S. Imaging tumor hypoxia by magnetic resonance methods. NMR Biomed. 2011, 24, 1–16. [Google Scholar] [CrossRef]
- Povich, M.J. Measurement of Dissolved Oxygen Concentrations and Diffusion Coefficients by Electron Spin Resonance. Anal. Chem. 1975, 47, 346–347. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- More, K.N.; Lim, T.H.; Kim, S.Y.; Kang, J.; Inn, K.S.; Chang, D.J. Characteristics of new bioreductive fluorescent probes based on the xanthene fluorophore: Detection of nitroreductase and imaging of hypoxic cells. Dyes Pigment. 2018, 151, 245–253. [Google Scholar] [CrossRef]
- Xia, L.; Hu, F.; Huang, J.; Li, N.; Gu, Y.; Wang, P. A fluorescent turn-on probe for nitroreductase imaging in living cells and tissues under hypoxia conditions. Sens. Actuators B Chem. 2018, 268, 70–76. [Google Scholar] [CrossRef]
- Gebremedhin, K.H.; Li, Y.; Yao, Q.; Xiao, M.; Gao, F.; Fan, J.; Du, J.; Long, S.; Peng, X. Development of a red-light emission hypoxia-sensitive two-photon fluorescent probe for: In vivo nitroreductase imaging. J. Mater. Chem. B 2019, 7, 408–414. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Liu, J.; Fu, J.; Sun, Y.; Ouyang, R.; Miao, Y. A near-infrared frequency upconversion probe for nitroreductase detection and hypoxia tumor in vivo imaging. Sens. Actuators B Chem. 2019, 286, 337–345. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, S.; Yoon, Y.; Lee, M.H. A resorufin-based fluorescent turn-on probe responsive to nitroreductase activity and its application to bacterial detection. Dyes Pigment. 2019, 171, 107779. [Google Scholar] [CrossRef]
- Kong, F.; Li, Y.; Yang, C.; Li, X.; Wu, J.; Liu, X.; Gao, X.; Xu, K.; Tang, B. A fluorescent probe for simultaneously sensing NTR and hNQO1 and distinguishing cancer cells. J. Mater. Chem. B 2019, 7, 6822–6827. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, L. Fluorescent probes for the selective detection of chemical species inside mitochondria. Chem. Commun. 2016, 52, 1094–1119. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, X.; Bright, S.A.; Taylor, N.C.; Elmes, R.B.P. 2-Nitroimidazole based fluorescent probes for nitroreductase; Monitoring reductive stress: In cellulo. Org. Biomol. Chem. 2017, 15, 6104–6108. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Xu, G.; Wang, R.; Zhu, T.; Tan, J.; Gu, X.; Zhao, C. Precise imaging of mitochondria in cancer cells by real-time monitoring of nitroreductase activity with a targetable and activatable fluorescent probe. Chem. Commun. 2020, 56, 7761–7764. [Google Scholar] [CrossRef]
- Yoon, S.A.; Chun, J.; Kang, C.; Lee, M.H. Self-Calibrating Bipartite Fluorescent Sensor for Nitroreductase Activity and Its Application to Cancer and Hypoxic Cells. ACS Appl. Bio Mater. 2021. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.; Jiang, W.; Dong, B.; Cui, H.; Tang, Y. Locked-flavylium fluorescent dyes with tunable emission wavelengths based on intramolecular charge transfer for multi-color ratiometric fluorescence imaging. Chem. Commun. 2015, 51, 6968–6971. [Google Scholar] [CrossRef]
- Cereda, E.; Turconi, M.; Ezhaya, A.; Brambilla, A.; Pagan, F.; Donetti, A. Anti-secretory and anti-ulcer activities of some new 2-(2-pyridylmethyl-sulfinyl)-benzimidazoles. Eur. J. Med. Chem. 1987, 22, 527–537. [Google Scholar] [CrossRef]
- Durchschein, K.; Hall, M.; Faber, K. Unusual reactions mediated by FMN-dependent ene- and nitro-reductases. Green Chem. 2013, 15, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, W.; Park, S.H.; Han, J.; Lee, J.; Kang, C.; Lee, M.H. An endoplasmic reticulum-selective ratiometric fluorescent probe for imaging a copper pool. Chem. Commun. 2017, 53, 4457–4460. [Google Scholar] [CrossRef]
- Koder, R.L.; Miller, A.F. Steady-state kinetic mechanism, stereospecificity, substrate and inhibitor specificity of Enterobacter cloacae nitroreductase. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1387, 395–405. [Google Scholar] [CrossRef]
- Preusch, P.C.; Siegel, D.; Gibson, N.W.; Ross, D. A note on the inhibition of DT-diaphorase by dicoumarol. Free Radic. Biol. Med. 1991, 11, 77–80. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, S.A.; Lee, M.H. Trimethyl Lock Quinone-Functionalized Coumarin for Real-time Monitoring of NQO1 Activity in the Live Cells. Bull. Korean Chem. Soc. 2021, 42, 119–123. [Google Scholar] [CrossRef]
- Dawson, R.C.M.; Elliot, D.C.; Elliot, W.H.; Jones, K.M. Data for Biochemical Research; Clarendon Press: London, UK, 1969. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Yoon, J.W.; Yoon, S.A.; Lee, M.H. Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe. Molecules 2021, 26, 1088. https://doi.org/10.3390/molecules26041088
Kim SJ, Yoon JW, Yoon SA, Lee MH. Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe. Molecules. 2021; 26(4):1088. https://doi.org/10.3390/molecules26041088
Chicago/Turabian StyleKim, Su Jung, Jung Won Yoon, Shin A Yoon, and Min Hee Lee. 2021. "Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe" Molecules 26, no. 4: 1088. https://doi.org/10.3390/molecules26041088
APA StyleKim, S. J., Yoon, J. W., Yoon, S. A., & Lee, M. H. (2021). Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe. Molecules, 26(4), 1088. https://doi.org/10.3390/molecules26041088