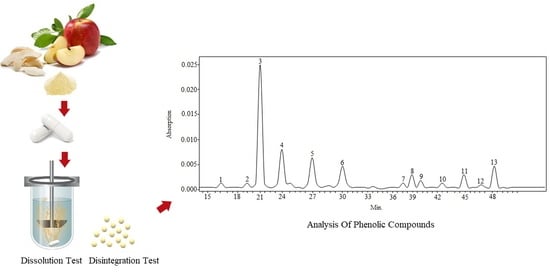
Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative Analysis of Phenolic Compounds of Apple Lyophilisate
2.2. Biopharmaceutical Evaluation of Hard Gelatin Capsules
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Solvents
3.3. Preparation of Apple Lyophilisate
3.4. Preparation of Phenolic Extracts
3.5. Qualitative and Quantitative Analysis by HPLC-PDA Method
3.6. Encapsulation Process
3.6.1. Test of the Uniformity of Mass of Single-Dose Preparations
3.6.2. Capsule Disintegration Test
3.6.3. Capsule Dissolution Test
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 23 May 2020).
- Marks, S.C.; Mullen, W.; Crozier, A. Flavonoid and chlorogenic acid profiles of English cider apples. J. Sci. Food Agric. 2007, 87, 719–728. [Google Scholar] [CrossRef]
- Price, K.; Prosser, T.; Richetin, A.; Rhodes, M. A comparison of the flavonol content and composition in dessert, cooking and cider-making apples; distribution within the fruit and effect of juicing. Food Chem. 1999, 66, 489–494. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Morresi, C.; Cianfruglia, L.; Armeni, T.; Mancini, F.; Tenore, G.C.; D’Urso, E.; Micheletti, A.; Ferretti, G.; Bacchetti, T. Polyphenolic compounds and nutraceutical properties of old and new apple cultivars. J. Food Biochem. 2018, 42, e12641. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2017, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Cantini, C.; Guarnieri, M.; Nepi, M.; Hausman, J.-F.; Guerriero, G.; Romi, M.; Cai, G. Nutraceutical Characteristics of Ancient Malus x domestica Borkh. Fruits Recovered across Siena in Tuscany. Medicines 2019, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2019, 246, 437–448. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a Source of Dietary Phytonutrients: Bioavailability and Evidence of Protective Effects against Human Cardiovascular Disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Nour, V.; Trandafir, I.; Ionica, M.E. Compositional characteristics of fruits of several apple (Malus domestica Borkh.) cultivars. Not. Bot. Hort. Agrobot. 2010, 38, 228–233. [Google Scholar]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus x domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, B.; Brinz, T.; Otterbach, S.; Khinast, J. Rapid automated process development of a continuous capsule-filling process. Int. J. Pharm. 2018, 546, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, P. Excipient selection for compounded pharmaceutical capsules: They’re only fillers, right? Aust. J. Pharm. 2017, 1164, 78–83. [Google Scholar]
- Nireesha, G.R.; Divya, L.; Sowmya, C.; Venkateshan, N.; Babu, M.N.; Lavakumar, V. Lyophilization/freeze drying—A review. IJNTPS 2013, 4, 87–98. [Google Scholar]
- Cock, L.S.; Munoz, D.P.V.; Aponte, A.A. Structural, physical, functional and nutraceutical changes of freeze-dried fruit. Afr. J. Biotechnol. 2015, 14, 442–450. [Google Scholar]
- Čakste, I.; Augšpole, I.; Cinkmanis, I.; Kuka, P. Bioactive Compounds in Latvian Wild Berry Juice. Mater. Sci. Appl. Chem. 2014, 30, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Bajića, A.; Pezo, L.L.; Stupara, A.; Filipčeva, B.; Cvetkovića, B.R.; Horeckic, A.T.; Mastilović, J. Application of lyophilized plum pomace as a functional ingredient in a plum spread: Optimizing texture, colour and phenol antioxidants by ANN modelling. LWT 2020, 130, 109588. [Google Scholar] [CrossRef]
- Delpino-Rius, A.; Eras, J.; Vilaró, F.; Cubero, M.Á.; Balcells, M.; Canela-Garayoa, R. Characterisation of phenolic compounds in processed fibres from the juice industry. Food Chem. 2015, 172, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowska, M.; Kropisz, P.; Wiśniewska, Z. Evaluation of the stability of the storage of selected fruit and vegetables freeze-dried powder based on the characteristics of the sorption properties. SJ GMU 2019, 19, 55–63. [Google Scholar]
- Jakobek, L.; Barron, A.R. Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, A.J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P.; Tuszy’nski, T. The profile of polyphenols and antioxidant properties of selected apple cultivars grown in Poland. J. Fruit Ornam. Plant Res. 2010, 18, 39–50. [Google Scholar]
- Iacopini, P.; Camangi, F.; Stefani, A.; Sebastiani, L. Antiradical potential of ancient Italian apple varieties of Malus x domestica Borkh. in a peroxynitrite-induced oxidative process. J. Food Compos. Anal. 2010, 23, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Bandoniene, D.; Murkovic, M. On-Line HPLC-DPPH Screening Method for Evaluation of Radical Scavenging Phenols Extracted from Apples (Malus domesticaL.). J. Agric. Food Chem. 2002, 50, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, H.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the Anti-inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; Pfeiffer, A.F.H. Foods for the prevention of diabetes: How do they work? Diabetes Metab. Res. Rev. 2012, 28, 25–49. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- Kanno, Y.; Watanabe, R.; Zempo, H.; Ogawa, M.; Suzuki, J.-I.; Isobe, M. Chlorogenic acid attenuates ventricular remodeling after myocardial infarction in mice. Int. Hear. J. 2013, 54, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Liu, C.-W.; Ma, Y.-S.; Weng, S.-W.; Tang, N.-Y.; Wu, S.-H.; Ji, B.-C.; Ma, C.-Y.; Ko, Y.-C.; Funayama, S.; et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. In Vivo. 2012, 26, 971–978. [Google Scholar]
- Cinkilic, N.; Çetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Çavaş, T.; Tunc, S.; Özkan, L.; Bilaloĝlu, R.; Yılmaz, D. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Han, J.; Hou, N.; Song, Y.; Dong, L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anti-Cancer Drugs 2015, 26, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Carnésecchi, S.; Schneider, Y.; A Lazarus, S.; Coehlo, D.; Gossé, F.; Raul, F. Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells. Cancer Lett. 2002, 175, 147–155. [Google Scholar] [CrossRef]
- Lotito, S.B.; Actis-Goretta, L.; Renart, M.; Caligiuri, M.; Rein, D.; Schmitz, H.H.; Steinberg, F.M.; Keen, C.L.; Fraga, C.G. Influence of Oligomer Chain Length on the Antioxidant Activity of Procyanidins. Biochem. Biophys. Res. Commun. 2000, 276, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.M.; Arola, L.; Ardèvol, A.; Blade, C.; Larrea, J.F.; Pujadas, G.; Salvadó, J.; et al. Grape-seed procyanidins act as anti-inflammatory agents in endotoxin stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Rocha, J.; Sepodes, B.; Matias, A.A.; Feliciano, R.P.; Carvalho, A.; Bronze, M.R.; Duarte, C.M.M.; Figueira, M.E. Evaluation of cardiovascular protective effect of different apple varieties correlation of response with composition. Food Chem. 2012, 135, 2378–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lina, B.; Reus, A.; Hasselwander, O.; Bui, Q.; Tenning, P. Safety evaluation of EvesseTM EPC, an apple polyphenol extract rich in flavan-3-ols. Food Chem. Toxicol. 2012, 50, 2845–2853. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Zhou, T.; Chen, G. Sulfation of dietary flavonoids by human sulfotransferases. Xenobiotica 2009, 39, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, E.L.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient apple cultivars from Garfagnana (Tuscany, Italy): A potential source for ‘nutrafruit’ production. Food Chem. 2019, 294, 518–525. [Google Scholar] [CrossRef]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Phloridzin reduces blood glucose levels and alters hepatic gene expression in normal BALB/c mice. Food Chem. Toxicol. 2012, 50, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 39, 5299–5306. [Google Scholar] [CrossRef]
- Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G. Novel Data on the Polyphenol Composition of Italian Ancient Apple Cultivars. Int. J. Food Prop. 2013, 16, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Elsakhawy, M.; Hassan, M. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Alves, L.A.; Silva, J.B.A.; Giulietti, M. Solubility of D-glucose in water and ethanol/water mixtures. J. Chem. Eng. Data. 2007, 52, 2166–2170. [Google Scholar] [CrossRef]
- Li, C.; Martini, L.G.; Ford, J.L.; Robe, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Supplement 6.1; European Pharmacopoeia; Council of Europe 2007: Strasbourg, France, 2007.
- Vyas, T.; Dash, R.P.; Anandjiwala, S.; Nivsarkar, M. Formulation and pharmacokinetic evaluation of hard gelatin capsule encapsulating lyophilized Vasa Swaras for improved stability and oral bioavailability of vasicine. Fitoterapia 2011, 82, 446–453. [Google Scholar] [CrossRef]
- Esmaeili, S.; Dayani, L.; Taheri, A.; Zolfaghari, B. Phytochemical standardization, formulation and evaluation of oral hard gelatin capsules from Pinus eldarica bark extract. Avicenna J. Phytomed. 2020, 11, 168–179. [Google Scholar]
- Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. The BCS: Where do we go from here? Pharm. Technol. 2001, 5, 68–76. [Google Scholar]
- Brown, C.K.; Friedel, H.D.; Barker, A.R.; Buhse, L.F.; Keitel, S.; Cecil, T.L.; Kraemer, J.; Morris, J.M.; Reppas, C.; Stickelmeyer, M.P.; et al. FIP/AAPS joint workshop report: Dissolution in vitro release testing of novel/special dosage forms. Pharm. Sci. Tech. 2011, 12, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Kressmann, S.; Biber, A.; Wonnemann, M.; Schug, B.; Blume, H.H.; Muller, W.E. Influence of pharmaceutical quality on the biovailability of active components from Ginkgo biloba preparations. J. Pharm. Pharmacol. 2002, 54, 1507–1514. [Google Scholar] [CrossRef]
- Taglioli, V.; Bilia, A.R.; Ghiara, C.; Mazzi, G.; Mercati, V.; Vincieri, F.F. Evaluation of the dissolution behaviour of some commercial herbal drugs and their preparations. Die Pharm. 2001, 56, 868–870. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated Knowledge about Polyphenols: Functions, Bioavailability, Metabolism, and Health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Appleton, J. Evaluating the bioavailability of isoquercetin. Nat. Med. J. 2010, 2, 1–6. [Google Scholar]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an Optimized HPLC Method for the Detection of Various Phenolic Compounds in Apples from Lithuanian Cultivars. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A Comparative Study of Phenolic Content in Apple Fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Kviklys, D.; Liaudanskas, M.; Janulis, V.; Viskelis, P.; Rubinskienė, M.; Lanauskas, J.; Uselis, N. Rootstock genotype determines phenol content in apple fruits. Plant, Soil Environ. 2014, 60, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Monograph: 2.9.5. Uniformity of Mass of Single-Dose Preparations; European Pharmacopoeia 6.0; Council of Europe 2007: Strasbourg, France, 2007.
- Monograph: 2.9.1. Disintegration of Tablets and Capsules; European Pharmacopoeia 6.0; Council of Europe 2007: Strasbourg, France, 2007.




| CC | AL, g | SDX, g | MCC, g | HPMC, g | HEC, g | GL, g | ST, g | TCM, g | FQ | MM, g |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | 0.100 | 0.001 | 0.019 | 0.050 | - | - | - | 0.170 | The mass is powdery, the capsule is filled completely | 0.171 |
| N2 | 0.100 | 0.001 | - | 0.069 | - | - | - | 0.170 | 0.171 | |
| N3 | 0.100 | 0.001 | - | - | - | 0.069 | - | 0.170 | 0.069 | |
| N4 | 0.100 | 0.001 | 0.069 | - | - | - | - | 0.170 | 0.171 | |
| N5 | 0.100 | 0.001 | - | - | - | - | 0.069 | 0.170 | 0.171 | |
| N6 | 0.100 | - | - | 0.070 | - | - | - | 0.170 | 0.172 | |
| N7 | 0.100 | - | - | - | 0.070 | - | - | 0.170 | 0.171 | |
| N8 | 0.100 | - | - | 0.100 | - | - | - | 0.200 | 0.202 | |
| N9 | 0.100 | - | - | 0.150 | - | - | - | 0.250 | 0.251 | |
| N10 | 0.100 | - | - | 0.250 | - | - | - | 0.350 | 0.349 | |
| N11 | 0.100 | - | - | 0.500 | - | - | - | 0.600 | 0.658 |
| Chlorogenic Acid | Rutin | Hyperoside | Isoquercitrin | Quercitrin | Avicularin | (−)-Epicatechin | Phloridzin | |
|---|---|---|---|---|---|---|---|---|
| CC | μg/mL | |||||||
| N1 | 372.5 B | 160.0 B | 144.5 B | 7.0 A | 97.5 B | 149.0 B | 17.5 A | 122.5 B |
| N2 | 367.5 B | 157.5 B | 140.0 B | 6.0 A | 89.5 B | 142.5 B | 9.5 B | 117.5 B |
| N3 | 372.5 B | 149.5 B | 139.0 B | 7.5 A | 91.5 B | 148.5 B | 10.0 B | 121.5 B |
| N4 | 367.5 B | 152.5 B | 140.5 B | 5.5 A | 92.5 B | 142.0 B | 14.5 A | 125.0 B |
| N5 | 385.0 B | 155.0 B | 132.5 B | 4.9 A | 87.5 B | 141.5 B | 8.5 B | 110.0 B |
| N6 | 589.5 A | 265.6 A | 240.4 A | 5.0 A | 170.7 A | 253.7 A | 6.3 B,C | 140.5 A |
| N7 | 640.3 A | 268.3 A | 241.8 A | 4.9 A | 166.5 A | 254.3 A | 10.2 B | 119.9 B |
| N8 | 167.9 C | 62.1 C | 60.9 C | 2.3 B | 40.3 C | 61.8 C | 1.2 D | 50.6 C |
| N9 | 128.3 C | 60.8 C | 59.6 C | 2.3 B | 39.4 C | 59.6 C | 1.1 D | 49.5 C |
| N10 | 126.4 C | 39.9 C | 49.4 C | 2.9 B | 33.3 C | 50.4 C | 3.8 C | 40.9 C |
| N11 | 68.4 D | 32.4 D | 31.7 D | 1.2 C | 21.0 D | 31.8 D | 0.6 D | 26.4 D |
| Release Content, % | Chlorogenic Acid | Rutin | Hyperoside | Isoquercitrin | Quercitrin | Avicularin | (−)-Epicatechin | Phloridzin |
|---|---|---|---|---|---|---|---|---|
| N1 | 96.0 A | 94.0 A | 88.0 A | 85.0 A | 80.0 A | 90.0 A | 83.0 A | 97.0 A |
| N2 | 94.0 A | 92.0 A | 87.0 A | 84.0 A | 82.0 A | 92.0 A | 80.0 A | 95.0 A |
| N3 | 95.0 A | 85.0 A | 89.0 A | 81.0 A | 80.0 A | 90.0 A | 81.0 A | 94.0 A |
| N4 | 97.0 A | 90.0 A | 89.0 A | 83.0 A | 85.0 A | 88.0 A | 83.0 A | 92.0 A |
| N5 | 94.0 A | 88.0 A | 90.0 A | 85.0 A | 80.0 A | 92.0 A | 85.0 A | 96.0 A |
| N6 | 95.0 A | 96.0 A | 90.0 A | 84.0 A | 84.5 A | 92.0 A | 81.0 A | 94.0 A |
| N7 | 90.0 A | 92.0 A | 88.0 A | 80.0 A | 83.0 A | 91.0 A | 82.0 A | 92.0 A |
| N8 | 55.0 B | 52.0 B | 53.0 B | 35.0 B | 38.0 B | 47.0 B | 35.0 B | 50.0 B |
| N9 | 52.0 B | 55.0 B | 56.0 B | 34.0 B | 37.0 B | 40.0 B | 34.0 B | 54.0 B |
| N10 | 50.0 B | 39.0 B | 42.0 B | 29.0 B | 30.0 B | 40.0 B | 29.0 B | 47.0 B |
| N11 | 29.0 C | 26.0 C | 29.0 C | 19.0 C | 18.0 C | 27.0 C | 19.0 C | 28.0 C |
| Release Content, % | Chlorogenic Acid | Rutin | Hyperoside | Isoquercitrin | Quercitrin | Avicularin | (−)-Epicatechin | Phloridzin |
|---|---|---|---|---|---|---|---|---|
| After 60 min | ||||||||
| N8 | 88.0 A | 85.0 A | 83.0 A | 76.0 A | 80.0 A | 83.0 A | 81.0 A | 86.0 A |
| N9 | 82.0 A | 82.0 A | 86.0 A | 79.0 A | 80.0 A | 81.0 A | 79.0 A | 82.0 A |
| N10 | 79.0 A | 74.0 A | 76.0 A | 70.0 A | 73.0 A | 77.0 A | 69.0 A | 78.0 A |
| N11 | 57.0 B | 55.0 B | 51.0 B | 45.0 B | 40.0 B | 57.0 B | 39.0 B | 55.0 B |
| After 75 min | ||||||||
| N8 | 90.0 A | 86.0 A | 87.0 A | 81.0 A | 82.0 A | 87.0 A | 83.0 A | 88.0 A |
| N9 | 91.0 A | 89.0 A | 88.0 A | 81.0 A | 83.0 A | 88.0 A | 82.0 A | 90.0 A |
| N10 | 88.0 A | 88.0 A | 86.0 A | 83.0 A | 82.0 A | 87.0 A | 81.0 A | 89.0 A |
| N11 | 92.0 A | 86.0 A | 87.0 A | 85.0 A | 83.0 A | 87.0 A | 81.0 A | 88.0 A |
| After 90 min | ||||||||
| N8 | 95.0 A | 92.0 A | 93.0 A | 89.0 A | 88.0 A | 91.0 A | 88.0 A | 96.0 A |
| N9 | 97.0 A | 94.0 A | 96.0 A | 92.0 A | 90.0 A | 93.0 A | 91.0 A | 96.0 A |
| N10 | 96.0 A | 95.0 A | 90.0 A | 89.0 A | 87.0 A | 90.0 A | 89.0 A | 97.0 A |
| N11 | 94.0 A | 93.0 A | 92.0 A | 88.0 A | 89.0 A | 91.0 A | 88.0 A | 93.0 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkevičiūtė, A.; Liaudanskas, M.; Ramanauskienė, K.; Janulis, V. Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules 2021, 26, 1095. https://doi.org/10.3390/molecules26041095
Butkevičiūtė A, Liaudanskas M, Ramanauskienė K, Janulis V. Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules. 2021; 26(4):1095. https://doi.org/10.3390/molecules26041095
Chicago/Turabian StyleButkevičiūtė, Aurita, Mindaugas Liaudanskas, Kristina Ramanauskienė, and Valdimaras Janulis. 2021. "Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder" Molecules 26, no. 4: 1095. https://doi.org/10.3390/molecules26041095
APA StyleButkevičiūtė, A., Liaudanskas, M., Ramanauskienė, K., & Janulis, V. (2021). Biopharmaceutical Evaluation of Capsules with Lyophilized Apple Powder. Molecules, 26(4), 1095. https://doi.org/10.3390/molecules26041095