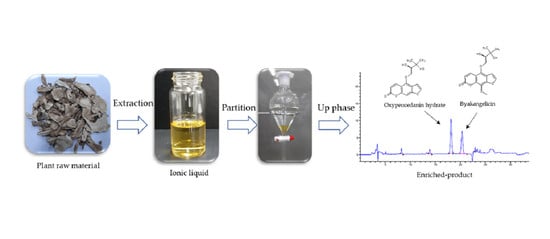
Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of ILs
2.2. Single Factor Experiments
2.2.1. Effect of the Solvent/Solid Ratio on the Extraction of Oxypeucedanin Hydrate and Byakangelicin
2.2.2. Effect of Temperature on the Extraction of Oxypeucedanin Hydrate and Byakangelicin
2.2.3. Effect of Time on the Extraction of Oxypeucedanin Hydrate and Byakangelicin
2.3. Statistical Optimization of the Extraction Conditions of Oxypeucedanin Hydrate and Byakangelicin
+ 1.6173X32 + 0.1489X1X2 + 0.2199X1X3 − 0.3581 X2X3
2.4. Extraction of Oxypeucedanin Hydrate and Byakangelicin Using Optimal Extraction Conditions
2.5. Isolation of Oxypeucedanin Hydrate and Byakangelicin from the IL Solution Using Back-Extraction
2.6. Comparison of the Extraction and Separation Method of Oxypeucedanin Hydrate and Byakangelicin
3. Materials and Methods
3.1. Reagents and Materials
3.2. Methods
3.2.1. Extraction Procedure and Quantitative Evaluation of Oxypeucedanin Hydrate and Byakangelicin in the IL Extraction Solutions
3.2.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.2.3. Quantitative Evaluation of Oxypeucedanin Hydrate and Byakangelicin
3.2.4. Isolation of Oxypeucedanin Hydrate and Byakangelicin from the IL Extraction Solution
3.2.5. Structural Identification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Bai, Y.; Li, D.; Zhou, T.; Qin, N.; Li, Z.; Yu, Z.; Hua, H. Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J. Funct. Foods 2016, 20, 453–462. [Google Scholar] [CrossRef]
- Deng, G.G.; Wei, W.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Gong, N.B.; Lü, Y.; Wang, F.F. New coumarins from the roots of Angelica dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO production in LPS-activated RAW264.7 cells. Fitoterapia 2015, 101, 194–200. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, J.; Zhang, L.; Gao, B.; Shi, S.; Wang, X.; Li, J.; Tu, P. Simultaneous Characterisation of Fifty Coumarins from the Roots of Angelica dahurica by Off-line Two-dimensional High-performance Liquid Chromatography Coupled with Electrospray Ionisation Tandem Mass Spectrometry. Phytochem. Anal. 2014, 25, 229–240. [Google Scholar] [CrossRef]
- Liao, Z.-G.; Liang, X.-L.; Zhu, J.-Y.; Zhao, G.-W.; Yang, M.; Wang, G.-F.; Jiang, Q.-Y.; Chen, X.-L. Correlation between synergistic action of Radix Angelica dahurica extracts on analgesic effects of Corydalis alkaloid and plasma concentration of dl-THP. J. Ethnopharmacol. 2010, 129, 115–120. [Google Scholar] [CrossRef]
- Yang, W.T.; Ke, C.Y.; Wu, W.T.; Tseng, Y.H.; Lee, R.P. Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in Staphylococcus aureus-infected wounds. Sci. Rep. 2020, 10, 5596. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Seo, C.S.; Lee, J.A.; Lee, N.H.; Kim, J.H.; Ha, H.; Zhen, M.-S.; Son, J.-K.; Shin, H.-K. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem. Toxicol. 2011, 49, 829–837. [Google Scholar] [CrossRef]
- Lee, K.; Shin, M.S.; Ham, I.; Choi, H.-Y. Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement. Altern. Med. 2015, 15, 395. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Hasnat, M.D.; Debnath, T.; Park, S.R.; Kim, D.H.; Lim, B.O. Antioxidant, anti-inflammatory and antiproliferative activity of Angelica dahurica root extracts. J. Food Biochem. 2014, 38, 281–292. [Google Scholar] [CrossRef]
- Marumoto, S.; Miyazawa, M. β-Secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother. Res. 2010, 24, 510–513. [Google Scholar] [CrossRef]
- Liang, W.-H.; Chang, T.-W.; Charng, Y.-C. Influence of harvest stage on the pharmacological effect of Angelica dahurica. Bot. Stud. 2018, 59, 14. [Google Scholar] [CrossRef]
- Chen, L.; Jian, Y.; Wei, N.; Yuan, M.; Zhuang, X.M.; Li, H. Separation and simultaneous quantification of nine furanocoumarins from Radix Angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci. 2015, 38, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Deng, R.; Zhou, L.; Meng, X.; Kuang, T.; Lai, X.; Zhang, J.; Zhang, Y. Development of a rapid resolution liquid chromatographic method combined with chemometrics for quality control of Angelica dahurica radix. Phytochem. Anal. 2012, 23, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, Y.; Wu, J.; Chen, X.; Hu, S.; Zhao, H.; Bai, X. Hollow fibre cell fishing and hollow fibre liquid phase microextraction research on the anticancer coumarins of Radix Angelicae dahuricae in vitro and in vivo. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 79–88. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.; Feng, Y.; Qiu, D. Simultaneous Determination of Three Coumarins in Angelica dahurica by 1H-qNMR Method: A Fast and Validated Method for Crude Drug Quality Control. J. Anal. Methods Chem. 2020, 2020, 8987560. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Peng, L.; Shi, M.; Li, C.; Zhang, Y.; Kang, W. Spectrum Effect Relationship and Component Knock-Out in Angelica Dahurica Radix by High Performance Liquid Chromatography-Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer. Molecules 2017, 22, 1231. [Google Scholar] [CrossRef] [Green Version]
- Amponsah, I.K.; Fleischer, T.C.; Dickson, R.A.; Annan, K.; Thoss, V. Evaluation of anti-inflammatory and antioxidant activity of Furanocoumarins and Sterolin from the stem bark of Ficus exasperata Vahl (Moraceae). J. Sci. Innov. Res. 2013, 2, 880–887. [Google Scholar]
- Kang, Y.Y.; Song, J.; Kim, J.Y.; Jung, H.; Yeo, W.-S.; Lim, Y.; Mok, H. Byakangelicin as a modulator for improved distribution and bioactivity of natural compounds and synthetic drugs in the brain. Phytomedicine 2019, 62, 152963. [Google Scholar] [CrossRef]
- Li, X.; Shao, S.; Li, H.; Bi, Z.; Zhang, S.; Wei, Y.; Bai, J.; Zhang, R.; Ma, X.; Ma, B.; et al. Byakangelicin protects against carbon tetrachloride–induced liver injury and fibrosis in mice. J. Cell. Mol. Med. 2020, 24, 8623–8635. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Ahrenberg, M.; Beck, M.; Neise, C.; Keßler, O.; Kragl, U.; Verevkin, S.P.; Schick, C. Vapor Pressure of Ionic Liquids at Low Temperatures from AC-Chip-Calorimetry. Phys. Chem. Chem. Phys. 2016, 18, 21381–21390. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Zhang, B.; Liu, S.H.; Chen, C.C. Flammability estimation of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Loss Prev. Process Ind. 2020, 66, 104196. [Google Scholar] [CrossRef]
- Villanueva, M.; Coronas, A.; García, J.; Salgado, J. Thermal Stability of Ionic Liquids for Their Application as New Absorbents. Ind. Eng. Chem. Res. 2013, 52, 15718–15727. [Google Scholar] [CrossRef]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Karmakar, A.; Mukundan, R.; Yang, P.; Batista, E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019, 9, 18506–18526. [Google Scholar] [CrossRef] [Green Version]
- Keaveney, S.T.; Haines, R.S.; Harper, J.B. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure Appl. Chem. 2017, 8, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in biotechnology and beyond. Solid State Ion. 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Mushtaq, M.; Akram, S. Ionic Liquid for the Extraction of Plant Phenolics. In Nanotechnology-Based Industrial Applications of Ionic Liquids; Nanotechnology in the Life Sciences; Inamuddin, A.A., Ed.; Spring: Cham, Switzerland, 2020; pp. 81–97. [Google Scholar]
- Bogdanov, M.G.; Svinyarov, I. Ionic liquid-supported solid–liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2013, 103, 279–288. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Y.; Hu, R.; Chen, J.; Zhang, Z.; Pan, Y. Application of ionic liquids-based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 2010, 80, 1292–1297. [Google Scholar] [CrossRef]
- Wang, L.; Bai, M.; Qin, Y.; Liu, B.; Wang, Y.; Zhou, Y. Application of Ionic Liquid-Based Ultrasonic-Assisted Extraction of Flavonoids from Bamboo Leaves. Molecules 2018, 23, 2309. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Wang, C. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2016, 21, 262. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef]
- Kiyonga, A.N.; An, J.H.; Lee, K.Y.; Lim, C.; Suh, Y.G.; Chin, Y.W.; Jung, K. Rapid and Efficient Separation of Decursin and Decursinol Angelate from Angelica gigas Nakai using Ionic Liquid, (BMIm)BF4, Combined with Crystallization. Molecules 2019, 24, 2390. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.A.R.; Domańska, U.; Schröder, B.; Coutinho, J.A.P.; Pinho, S.P. Selection of Ionic Liquids to be Used as Separation Agents for Terpenes and Terpenoids. ACS Sustain. Chem. Eng. 2015, 4, 548–556. [Google Scholar] [CrossRef]
- Ji, S.; Wang, Y.; Su, Z.; He, D.; Du, Y.; Guo, M.; Yang, D.; Tang, D. Ionic liquids-ultrasound based efficient extraction of flavonoid glycosides and triterpenoid saponins from licorice. RSC Adv. 2018, 8, 13989–13996. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Spronen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Mazzola, P.G.; Lopes, A.M.; Hasmann, F.A.; Jozala, A.F.; Penna, T.C.V.; Magalhaes, P.O.; Rangel-Yagui, C.O.; Pessoa, A. Liquid–liquid extraction of biomolecules: An overview and update of the main techniques. J. Chem. Technol. Biotechnol. 2008, 83, 143–157. [Google Scholar] [CrossRef]
- Absalan, G.; Akhond, M.; Sheikhian, L. Extraction and high performance liquid chromatographic determination of 3-indole butyric acid in pea plants by using imidazolium-based ionic liquids as extractant. Talanta 2008, 77, 407–411. [Google Scholar] [CrossRef]
- Larriba, M.; Omar, S.; Navarro, P.; Garcia, J.; Rodriguez, F.; Gonzalez-Miquel, M. Recovery of tyrosol from aqueous streams using hydrophobic ionic liquids: A first step towards developing sustainable processes for olive mill wastewater (OMW) management. RSC Adv. 2016, 6, 18751–18762. [Google Scholar] [CrossRef]
- Nazet, A.; Sokolov, S.; Sonnleitner, T.; Makino, T.; Kanakubo, M.; Buchner, R. Densities, Viscosities, and Conductivities of the Imidazolium Ionic Liquids [Emim][Ac], [Emim][FAP], [Bmim][BETI], [Bmim][FSI], [Hmim][TFSI], and [Omim][TFSI]. J. Chem. Eng. Data 2015, 60, 2400–2411. [Google Scholar] [CrossRef]
- Fan, W.; Zhou, Q.; Sun, J.; Zhang, S. Density, Excess Molar Volume, and Viscosity for the Methyl Methacrylate +1-Butyl-3-methylimidazolium Hexafluorophosphate Ionic Liquid Binary System at Atmospheric Pressure. J. Chem. Eng. Data 2009, 54, 2307–2311. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Zu, Y.-G.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem. Eng. J. 2011, 172, 705–712. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [Green Version]
- Naert, P.; Rabaey, K.; Stevens, C.V. Ionic liquid ion exchange: Exclusion from strong interactions condemns cations to the most weakly interacting anions and dictates reaction equilibrium. Green Chem. 2018, 20, 4277. [Google Scholar] [CrossRef]
- Ishihara, K.; Fukutake, M.; Asano, T.; Mizuhara, Y.; Wakui, Y.; Yanagisawa, T.; Kamei, H.; Ohmori, S.; Kitada, M. Simultaneous determination of byak-angelicin and oxypeucedanin hydrate in rat plasma by column-switching high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 2001, 753, 309–314. [Google Scholar] [CrossRef]









| Levels | X1 Extraction Time (min) | X2 Extraction Temperature (°C) | X3 Solvent/Solid Ratio (mL·g−1) |
|---|---|---|---|
| Low level (−1) | 60 | 40 | 6:1 |
| Average (0) | 120 | 50 | 9:1 |
| High level (1) | 180 | 60 | 12:1 |
| Run | X1 Extraction Time (min) | X2 Extraction Temperature (°C) | X3 Solvent/Solid Ratio (mL·g−1) | Extraction Yield of Oxypeucedanin Hydrate (%) | Extraction Yield of Byakangelicin (%) |
|---|---|---|---|---|---|
| 1 | 120 | 50 | 9:1 | 87.55 | 88.54 |
| 2 | 120 | 50 | 9:1 | 88.01 | 89.07 |
| 3 | 180 | 60 | 9:1 | 100.20 | 101.19 |
| 4 | 180 | 50 | 6:1 | 90.74 | 89.56 |
| 5 | 120 | 60 | 12:1 | 97.88 | 99.06 |
| 6 | 60 | 50 | 6:1 | 77.21 | 77.94 |
| 7 | 120 | 50 | 9:1 | 86.36 | 90.87 |
| 8 | 120 | 60 | 6:1 | 88.64 | 85.59 |
| 9 | 60 | 50 | 12:1 | 85.82 | 88.09 |
| 10 | 180 | 40 | 9:1 | 91.86 | 92.22 |
| 11 | 60 | 60 | 9:1 | 82.10 | 89.66 |
| 12 | 180 | 50 | 12:1 | 100.23 | 100.65 |
| 13 | 120 | 40 | 6:1 | 79.74 | 77.89 |
| 14 | 60 | 40 | 9:1 | 74.35 | 76.43 |
| 15 | 120 | 40 | 12:1 | 90.41 | 90.09 |
| Degrees of Freedom | Sum of Squares | Mean Square | F-Value | p-Value | R2 | R2 Adjusted | |
|---|---|---|---|---|---|---|---|
| Extraction yield of oxypeucedanin hydrate (%) | |||||||
| Model | 9 | 828.83 | 92.09 | 49.90 | 0.00 | ||
| X1 | 1 | 504.87 | 504.87 | 147.58 | 0.00 | ||
| X2 | 1 | 131.72 | 131.725 | 71.37 | 0.00 | ||
| X3 | 1 | 180.57 | 180.57 | 97.83 | 0.00 | ||
| X12 | 1 | 1.16 | 0.67 | 0.36 | 0.57 | ||
| X22 | 1 | 0.05 | 0.22 | 0.12 | 0.74 | 0.989 | 0.969 |
| X32 | 1 | 9.66 | 9.66 | 5.23 | 0.07 | ||
| X1 X2 | 1 | 0.09 | 0.09 | 0.05 | 0.83 | ||
| X1 X3 | 1 | 0.19 | 0.19 | 0.10 | 0.76 | ||
| X2 X3 | 1 | 0.51 | 0.51 | 0.28 | 0.62 | ||
| Residual | 5 | 9.23 | 1.85 | ||||
| Lack of fit | 3 | 7.78 | 2.59 | 3.58 | 0.23 | ||
| Pure error | 2 | 1.45 | 0.724 | ||||
| Total | 14 | 838.06 | |||||
| Extraction yield of byakangelicin (%) | |||||||
| Model | 9 | 807.01 | 89.67 | 42.82 | 0.00 | ||
| X1 | 1 | 331.42 | 331.42 | 158.25 | 0.00 | ||
| X2 | 1 | 188.95 | 188.95 | 90.22 | 0.00 | ||
| X3 | 1 | 275.08 | 275.08 | 131.35 | 0.00 | ||
| X1 | 1 | 2.01 | 1.50 | 0.72 | 0.43 | ||
| X22 | 1 | 0.12 | 0.26 | 0.12 | 0.74 | ||
| X32 | 1 | 4.28 | 4.28 | 2.04 | 0.21 | 0.987 | 0.964 |
| X1 X2 | 1 | 4.53 | 4.53 | 2.16 | 0.20 | ||
| X1 X3 | 1 | 0.22 | 0.22 | 0.11 | 0.76 | ||
| X2 X3 | 1 | 0.40 | 0.40 | 0.19 | 0.68 | ||
| Residual | 5 | 10.47 | 2.09 | ||||
| Lack of fit | 3 | 7.48 | 2.49 | 1.67 | 0.40 | ||
| Pure error | 2 | 2.99 | 1.49 | ||||
| Total | 14 | 817.48 | |||||
| Predicted Yield of Oxypeucedanin Hydrate (%) | Observed Yield of Oxypeucedanin Hydrate (%) | Predicted Yield of Byakangelicin (%) | Observed Yield of Byakangelicin (%) | |
|---|---|---|---|---|
| Mean | 97.99 | 98.06 | 98.01 | 99.52 |
| Standard Deviation | 2.37 | 0.96 | ||
| Relative Standard Deviation (%) | 2.42 | 0.96 |
| Back-Extraction Solvent | Recovered Amount of Oxypeucedanin Hydrate (μg) | Recovered Amount of Byakangelicin (μg) | Yield of Oxypeucedanin Hydrate (%) | Yield of Byakangelicin (%) |
|---|---|---|---|---|
| DIW | 332.15 | 300.50 | 54.46 | 41.18 |
| 0.01 N HCl | 517.79 | 570.98 | 84.90 | 78.24 |
| 0.01 NaOH | 0 | 0 | 0 | 0 |
| Volume of 0.01 N HCl (mL) | Recovered Amount of Oxypeucedanin Hydrate (μg) | Recovered Amount of Byakangelicin (μg) | Yield of Oxypeucedanin Hydrate (%) | Yield of Byakangelicin (%) |
|---|---|---|---|---|
| 10 | 342.22 | 257.22 | 56.11 | 35.24 |
| 20 | 441.64 | 538.66 | 72.41 | 73.81 |
| 30 | 529.42 | 580.29 | 86.81 | 79.51 |
| 40 | 561.06 | 650.762 | 91.99 | 89.17 |
| 50 | 541.69 | 660.012 | 89.47 | 90.44 |
| Recovered Amount (μg) | Yield (%) | Content (%) | |
|---|---|---|---|
| Total extract | 14,456.80 | - | - |
| Oxypeucedanin hydrate | 5347.09 | 90.17 | 36.99 |
| Byakangelicin | 6523.39 | 88.51 | 45.12 |
| Extraction Solvent | Amount of Oxypeucedanin Hydrate (μg) | Amount of Byakangelicin (μg) | Extraction Yield of Oxypeucedanin Hydrate (%) | Extraction Yield of Byakangelicin (%) |
|---|---|---|---|---|
| DIW | 462.42 | 625.01 | 37.90 | 41.39 |
| 50% ethanol | 885.39 | 782.64 | 72.57 | 51.83 |
| 95% ethanol | 946.18 | 1288.83 | 77.56 | 85.35 |
| [Bmim]Tf2N | 1194.89 | 1498.55 | 98.06 | 99.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyonga, A.N.; Hong, G.; Kim, H.S.; Suh, Y.-G.; Jung, K. Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid. Molecules 2021, 26, 830. https://doi.org/10.3390/molecules26040830
Kiyonga AN, Hong G, Kim HS, Suh Y-G, Jung K. Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid. Molecules. 2021; 26(4):830. https://doi.org/10.3390/molecules26040830
Chicago/Turabian StyleKiyonga, Alice Nguvoko, Gyeongmin Hong, Hyun Su Kim, Young-Ger Suh, and Kiwon Jung. 2021. "Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid" Molecules 26, no. 4: 830. https://doi.org/10.3390/molecules26040830
APA StyleKiyonga, A. N., Hong, G., Kim, H. S., Suh, Y. -G., & Jung, K. (2021). Facile and Rapid Isolation of Oxypeucedanin Hydrate and Byakangelicin from Angelica dahurica by Using [Bmim]Tf2N Ionic Liquid. Molecules, 26(4), 830. https://doi.org/10.3390/molecules26040830