Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells
Abstract
:1. Introduction
2. Results
2.1. Antioxidant and Neuroprotective Effects of Catechins
In Vitro Antioxidant Effect of Catechins
2.2. Neuroprotective Effects of Catechins against Glutamate-Induced Apoptosis in HT22 Cells
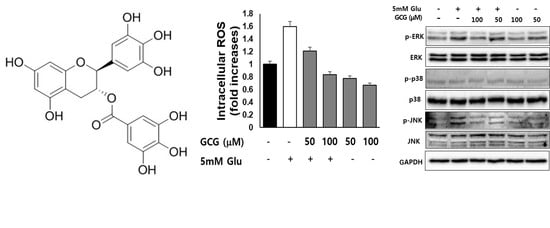
2.3. Effect of GCG on ROS Production by Glutamate in HT22 Cells
2.4. Effect of GCG on Glutamate-Induced Ca2+ Accumulation in HT22 Cells
2.5. Effect of GCG on Glutamate-Induced Nuclear Condensation in HT22 Cells
2.6. Effect of GCG on Glutamate-Induced Phosphorylation of MAPKs in HT22 Cells
3. Discussion
4. Materials and Methods
4.1. Antioxidant and Neuroprotective Effects of Catechins
4.2. Evaluation of DPPH Scavenging Ability of Catechins
4.3. Neuroprotective Effect of Catechins against Glutamate-Induced Excitotoxicity
4.3.1. Evaluation of Cell Viability
4.3.2. Evaluation of Neuroprotective Effects of Catechins in HT22 Cells against Glutamate Excitotoxicity
4.3.3. Determination of ROS Levels
4.3.4. Measurement of Ca2+ Levels
4.3.5. Evaluation of Nuclear Condensation Using Hoechst 33342 Reagent
4.3.6. Protein Separation (Preparation of Whole-Cell Lysates)
4.3.7. SDS-Polyacrylamide Gel Electrophoresis and Western Blot Analysis
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Qin, L.; Gao, H.; Wilson, B.; Ali, S.F.; Zhang, W.; Hong, J.; Liu, B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: Role of NADPH oxidase. FASEB J. 2004, 18, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free. Radic. Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Fukui, M.; Song, J.-H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of glutamate-Induced neurotoxicity in HT22 mouse hippocampal cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kerksick, C.M.; Willoughby, D.S. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Tóth, F.; Cseh, E.K.; Vécsei, L.. Natural molecules and neuroprotection: Kynurenic acid, pantethine and alpha-lipoic acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Kong, X.; Guan, J.; Gong, S.; Wang, R. Neuroprotective effects of grape seed procyanidin extract on ischemia-reperfusion brain injury. Chin. Med. Sci. J. 2017, 32, 92–99. [Google Scholar]
- Ikeda, A.; Iso, H.; Yamagishi, K.; Iwasaki, M.; Yamaji, T.; Miura, T.; Sawada, N.; Inoue, M.; Tsugane, S.; Miura, T. Plasma tea catechins and risk of cardiovascular disease in middle-aged Japanese subjects: The JPHC study. Atherosclerosis 2018, 277, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, J.; Sun, X.; Shi, X.; Wang, L.; Huang, L.; Zhou, W. Evaluation of the neuroprotective effect of EGCG: A potential mechanism of mitochondrial dysfunction and mitochondrial dynamics after subarachnoid hemorrhage. Food Funct. 2018, 9, 6349–6359. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Maher, P.; van Leyen, K.; Dey, P.N.; Honrath, B.; Dolga, A.; Methner, A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 2018, 70, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Starkov, A.A.; Chinopoulos, C.; Fiskum, G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 2004, 36, 257–264. [Google Scholar] [CrossRef]
- Parfenova, H.; Basuroy, S.; Bhattacharya, S.; Tcheranova, D.; Qu, Y.; Regan, R.F.; Leffler, C.W. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: Contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol. Physiol. 2006, 290, C1399–C1410. [Google Scholar] [CrossRef]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Hao, L.; Wang, H.-Y.; Sun, Y.-J.; Yang, X.-Y.; Che, B.; Xue, J.; Gao, Z.-B. Neuroprotective Effect of Resveratrol Against Glutamate-Induced Excitotoxicity. Adv. Clin. Exp. Med. 2015, 24, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement. Altern. Med. 2020, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Koo, M.W.L. EGCG protects HT-22 cells against glutamate-induced oxidative stress. Neurotox. Res. 2006, 10, 23–29. [Google Scholar] [CrossRef]
- Cong, L.; Cao, C.; Cheng, Y.; Qin, X.-Y. Green Tea Polyphenols Attenuated Glutamate Excitotoxicity via Antioxidative and Antiapoptotic Pathway in the Primary Cultured Cortical Neurons. Oxidative Med. Cell. Longev. 2015, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Eldeiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2008, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Weon, J.B.; Yang, W.S.; Ryu, G.; Ma, C.J. Neuroprotective effects of Magnoliae Flos extract in mouse hippocampal neuronal cells. Sci. Rep. 2018, 8, 9693. [Google Scholar] [CrossRef]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic. Biol. Med. 2010, 49, 800–813. [Google Scholar] [CrossRef] [Green Version]
- Pallast, S.; Arai, K.; Wang, X.; Lo, E.H.; Van Leyen, K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J. Neurochem. 2009, 111, 882–889. [Google Scholar] [CrossRef] [Green Version]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Pearson, G.; Robinson, F.; Gibson, T.B.; Xu, B.-E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions *. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, S.M.; Connolly, C.N. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology 2007, 53, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kook, S.-H.; Ji, H.; Lee, S.-A.; Choi, K.-C.; Lee, K.-Y.; Lee, J.-C. N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway. BMB Rep. 2015, 48, 636–641. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Cheng, H.; Su, J.; Wang, X.; Wang, Q.; Chu, J.; Li, Q. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol. In Vitro 2020, 62, 104715. [Google Scholar] [CrossRef] [PubMed]
- Posod, A.; Winkler, I.; Wegleiter, K.; Huber, E.; Urbanek, M.; Kiechl-Kohlendorfer, U.; Griesmaier, E. The effect of levomepromazine on the healthy and injured developing mouse brain—An in vitro and in vivo study. IBRO Rep. 2020, 9, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bonde, C.; Noraberg, J.; Zimmer, J. Nuclear shrinkage and other markers of neuronal cell death after oxygen–glucose deprivation in rat hippocampal slice cultures. Neurosci. Lett. 2002, 327, 49–52. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Choi, E.; Kim, E.; Kim, J.H.; Yoon, K.; Kim, S.; Lee, J.; Cho, J.Y. AKT1-targeted proapoptotic activity of compound K in human breast cancer cells. J. Ginseng Res. 2019, 43, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng Res. 2019, 43, 326–334. [Google Scholar] [CrossRef]
- Lim, L.; Ju, S.; Song, H. Dendropanax morbifera Extract Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury by Inhibition of Reactive Oxygen Species Generation and Calcium Perturbation. Nat. Prod. Sci. 2019, 25. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Zhou, C.; Li, Y.; Duan, W.; Zhang, B.; Wang, M.; Fang, J. 20(S)-Ginsenoside Rh2 displays efficacy against T-cell acute lymphoblastic leukemia through the PI3K/Akt/mTOR signal pathway. J. Ginseng Res. 2020, 44, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, M. Anti-inflammatory effects of fermented products with Avena sativa on RAW264.7 and HT-29 cells via inhibition of inflammatory mediators. Nat. Prod. Sci. 2020, 26, 244–251. [Google Scholar]






| Relative Protection (%) | ||
|---|---|---|
| Concentration (μM) | GCG | 5 mM Glu + GCG |
| 0 | 100.00 ± 2.81 | 32.16 ± 3.01 |
| 3.125 | 95.54 ± 4.21 | 34.04 ± 4.53 |
| 6.25 | 95.87 ± 3.89 | 37.77 ± 3.21 |
| 12.5 | 96.80 ± 3.28 | 33.14 ± 4.47 |
| 25 | 94.62 ± 4.52 | 30.21 ± 4.25 |
| 50 | 94.87 ± 2.31 | 52.03 ± 2.31 |
| 100 | 96.57 ± 3.57 | 96.80 ± 3.57 |
| 200 | 94.67 ± 4.57 | 94.18 ± 2.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.H.; Park, J.Y.; Kang, K.S.; Hwang, G.S. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2021, 26, 1387. https://doi.org/10.3390/molecules26051387
Park DH, Park JY, Kang KS, Hwang GS. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules. 2021; 26(5):1387. https://doi.org/10.3390/molecules26051387
Chicago/Turabian StylePark, Do Hwi, Jun Yeon Park, Ki Sung Kang, and Gwi Seo Hwang. 2021. "Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells" Molecules 26, no. 5: 1387. https://doi.org/10.3390/molecules26051387
APA StylePark, D. H., Park, J. Y., Kang, K. S., & Hwang, G. S. (2021). Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules, 26(5), 1387. https://doi.org/10.3390/molecules26051387