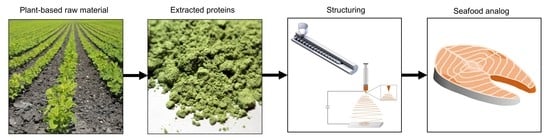
Plant-Based Seafood Analogs
Abstract
:1. Introduction
2. The Objects of Imitation: Fish, Surimi and Other Seafood Products
2.1. Fish Flesh
2.2. Surimi
2.3. Processed Fish Products and Surimi-Based Products
3. Textural and Sensorial Properties of Fish
3.1. Texture
3.2. Appearance
3.3. Flavor
4. Plant-Based Proteins (PBP) Used to Mimic Muscle Structure and Texture
5. Non-Protein Texture-Functional Ingredients in Fish Analogs
5.1. Ions
5.2. Lipids
5.3. Dietary Fibers
6. Structuring Techniques
6.1. Extrusion
6.2. Electrospinning
6.3. Wet Spinning
6.4. 3D Printing
7. Conclusions
Funding
Conflicts of Interest
References
- McClements, D.J. Future foods: A manifesto for research priorities in structural design of foods. Food Funct. 2020, 11, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; Declerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Uddin, E.; Kebreab, E. Review: Impact of Food and Climate Change on Pastoral Industries. Front. Sustain. Food Syst. 2020, 4, 543403. [Google Scholar] [CrossRef]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and Cancer Risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.E.; Higuchi, A. Is fish worth more than meat? How consumers’ beliefs about health and nutrition affect their willingness to pay more for fish than meat. Food Qual. Preference 2018, 65, 101–109. [Google Scholar] [CrossRef]
- Almeida, C.; Karadzic, V.; Vaz, S. The seafood market in Portugal: Driving forces and consequences. Mar. Policy 2015, 61, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Dowgiałło, A.; Stachnik, M.; Grochowicz, J.; Jakubowski, M. Modeling of compression pressure of heated raw fish during pressing liquid. J. Food Eng. 2020, 276, 109888. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Sunderland, E.M.; Chan, H.M.; Choi, A.L.; Grandjean, P.; Mariën, K.; Oken, E.; Sakamoto, M.; Schoeny, R.; Weihe, P.; et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr. Rev. 2011, 69, 493–508. [Google Scholar] [CrossRef]
- Oken, E.; Choi, A.L.; Karagas, M.R.; Mariën, K.; Rheinberger, C.M.; Schoeny, R.; Sunderland, E.; Korrick, S. Which Fish Should I Eat? Perspectives Influencing Fish Consumption Choices. Environ. Health Perspect. 2012, 120, 790–798. [Google Scholar] [CrossRef]
- Yaktine, A.L.; Nesheim, M.C. Seafood Choices: Balancing Benefits and Risks; National Academies Press: Washington, DC, USA, 2007; ISBN 0309102189. [Google Scholar]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Thavamani, A.; Sferra, T.J.; Sankararaman, S. Meet the Meat Alternatives: The Value of Alternative Protein Sources. Curr. Nutr. Rep. 2020, 9, 346–355. [Google Scholar] [CrossRef]
- Van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-Based Meats, Human Health, and Climate Change. Front. Sustain. Food Syst. 2020, 4, 128. [Google Scholar] [CrossRef]
- Nisov, A.; Aisala, H.; Holopainen-Mantila, U.; Alakomi, H.-L.; Nordlund, E.; Honkapää, K. Comparison of Whole and Gutted Baltic Herring as a Raw Material for Restructured Fish Product Produced by High-Moisture Extrusion Cooking. Foods 2020, 9, 1541. [Google Scholar] [CrossRef] [PubMed]
- Borderías, A.J.; Tovar, C.A.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by pea protein isolates. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT 2013, 50, 511–518. [Google Scholar] [CrossRef]
- Luo, Y.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Effect of soy protein isolate on gel properties of Alaska pollock and common carp surimi at different setting conditions. J. Sci. Food Agric. 2004, 84, 663–671. [Google Scholar] [CrossRef]
- Venugopal, V.; Shahidi, F. Structure and composition of fish muscle. Food Rev. Int. 1996, 12, 175–197. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Han, Z.; Zeng, X.-A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 13, 52–61. [Google Scholar] [CrossRef]
- Chéret, R.; Chapleau, N.; de Lamballerie, M.; Delbarre-Ladrat, C.; Verrez-Bagnis, V. Effects of High Pressure on Texture and Microstructure of Sea Bass (Dicentrarchus labrax L.) Fillets. J. Food Sci. 2005, 70, e477–e483. [Google Scholar] [CrossRef] [Green Version]
- Aussanasuwannakul, A.; Slider, S.D.; Salem, M.; Yao, J.; Kenney, P.B. Comparison of Variable-Blade to Allo-Kramer Shear Method in Assessing Rainbow Trout (Oncorhynchus mykiss) Fillet Firmness. J. Food Sci. 2012, 77, S335–S341. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Venugopal, V. Seafood Processing: Adding Value through Quick Freezing, Retortable Packaging and Cook-Chilling; CRC Press: Boca Raton, FL, USA, 2005; ISBN 1420027395. [Google Scholar]
- Tahergorabi, R.; Jaczynski, J. Physicochemical changes in surimi with salt substitute. Food Chem. 2012, 132, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.M.; Herranz, B.; Pérez-Mateos, M.; Sánchez-Alonso, I.; Borderías, J.A.; Borderías, A.J. New Alternatives in Seafood Restructured Products. Crit. Rev. Food Sci. Nutr. 2013, 56, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Yongsawatdigul, J.; Carvajal, P.; Lanier, T. Surimi Gelation Chemistry. In Food Plant Economics; CRC Press: Boca Raton, FL, USA, 2005; Volume 2, pp. 435–489. [Google Scholar]
- Boziaris, I.S. Seafood Processing: Technology, Quality and Safety; Boziaris, I.S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; ISBN 9781118346174. [Google Scholar]
- Moreno, H.M.; Carballo, J.; Borderías, A.J. Influence of alginate and microbial transglutaminase as binding ingredients on restructured fish muscle processed at low temperature. J. Sci. Food Agric. 2008, 88, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Veland, J.O.; Torrissen, O.J. The texture of Atlantic salmon (Salmo salar) muscle as measured instrumentally using TPA and Warner-Brazier shear test. J. Sci. Food Agric. 1999, 79, 1737–1746. [Google Scholar] [CrossRef]
- Bland, J.M.; Bett-Garber, K.L.; Li, C.H.; Brashear, S.S.; Lea, J.M.; Bechtel, P.J. Comparison of sensory and instrumental methods for the analysis of texture of cooked individually quick frozen and fresh-frozen catfish fillets. Food Sci. Nutr. 2018, 6, 1692–1705. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Maung, T.-T.; Gu, B.-Y.; Ryu, G.-H. Influence of extrusion process parameters on specific mechanical energy and physical properties of high-moisture meat analog. Int. J. Food Eng. 2021, 17, 149–157. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; Eijnden, P.V.D.; Henket, J.T.; Langelaan, M.L.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Food-grade electrospinning of proteins. Innov. Food Sci. Emerg. Technol. 2013, 20, 269–275. [Google Scholar] [CrossRef]
- Casas, C.; Martinez, O.; Guillen, M.; Pin, C.; Salmeron, J. Textural properties of raw Atlantic salmon (Salmo salar) at three points along the fillet, determined by different methods. Food Control 2006, 17, 511–515. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W.; Zhang, X.; Li, X.; Zhao, J. Shelf-life extension of crucian carp (Carassius auratus) using natural preservatives during chilled storage. Food Chem. 2012, 135, 140–145. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W.; He, Y. Novel non-invasive distribution measurement of texture profile analysis (TPA) in salmon fillet by using visible and near infrared hyperspectral imaging. Food Chem. 2014, 145, 417–426. [Google Scholar] [CrossRef]
- Isaksson, T.; Swensen, L.P.; Taylor, R.G.; Fjaera, S.O.; Skjervold, P.O. Non-destructive texture analysis of farmed Atlantic salmon using visual/near-infrared reflectance spectroscopy. J. Sci. Food Agric. 2001, 82, 53–60. [Google Scholar] [CrossRef]
- Li, C.H.; Bland, J.M.; Bechtel, P.J. Effect of precooking and polyphosphate treatment on the quality of microwave cooked catfish fillets. Food Sci. Nutr. 2017, 5, 812–819. [Google Scholar] [CrossRef]
- Pavón, Y.; Cian, R.E.; Soldini, M.A.C.; Hernández, D.R.; Sánchez, S.; Drago, S.R. Sensory and instrumental textural changes in fillets from Pacú (Piaractus mesopotamicus) fed different diets. J. Texture Stud. 2018, 49, 646–652. [Google Scholar] [CrossRef]
- Soares, N.; da Silva, P.M.P.; Barbosa, C.; Pinheiro, R.; Vicente, A.A. Comparing the effects of glazing and chitosan-based coating applied on frozen salmon on its organoleptic and physicochemical characteristics over six-months storage. J. Food Eng. 2017, 194, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Effect of partial sodium replacement on physicochemical parameters of smoked sea bass during storage. Food Sci. Technol. Int. 2012, 18, 207–217. [Google Scholar] [CrossRef]
- Santana, P.; Huda, N.; Yang, T.A. Physicochemical properties and sensory characteristics of sausage formulated with surimi powder. J. Food Sci. Technol. 2013, 52, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Quadros, D.A.; Rocha, I.F.D.O.; Ferreira, S.M.R.; Bolini, H.M.A. Low-sodium fish burgers: Sensory profile and drivers of liking. LWT 2015, 63, 236–242. [Google Scholar] [CrossRef]
- Shahidi, F.; Botta, J.R. Seafoods: Chemistry, Processing Technology and Quality; Springer Science & Business Media: Dordecht, Germany, 2012; ISBN 1461521815. [Google Scholar]
- Baten, M.A.; Won, N.E.; Mohibbullah, M.; Yoon, S.J.; Hak, S.J.; Kim, J.S.; Choi, J.S. Effect of hot smoking treatment in improving Sensory and Physicochemical Properties of processed Japanese Spanish Mackerel Scomberomorus niphonius. Food Sci. Nutr. 2020, 8, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Ebeneezar, S.; Prabu, D.L.; Chandrasekar, S.; Tejpal, C.; Madhu, K.; Sayooj, P.; Vijayagopal, P. Evaluation of dietary oleoresins on the enhancement of skin coloration and growth in the marine ornamental clown fish, Amphiprion ocellaris (Cuvier, 1830). Aquaculture 2020, 529, 735728. [Google Scholar] [CrossRef]
- Hyldig, G.; Nielsen, D. A Review of Sensory and Instrumental Methods Used to Evaluate the Texture of Fish Muscle. J. Texture Stud. 2001, 32, 219–242. [Google Scholar] [CrossRef]
- Ninomiya, K. Umami: A universal taste. Food Rev. Int. 2002, 18, 23–38. [Google Scholar] [CrossRef]
- Vajdi, M.; Varidi, M.J.; Varidi, M.; Mohebbi, M. Using electronic nose to recognize fish spoilage with an optimum classifier. J. Food Meas. Charact. 2019, 13, 1205–1217. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Nadathur, S.; Wanasundara, J.P.D.; Scanlin, L. Sustainable Protein Sources; Academic Press Elsevier: Amsterdam, The Netherlands, 2016; ISBN 0128027762. [Google Scholar]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6—Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. ISBN 978-0-12-814874-7. [Google Scholar]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Guo, Z.; Teng, F.; Huang, Z.; Lv, B.; Lv, X.; Babich, O.; Yu, W.; Li, Y.; Wang, Z.; Jiang, L. Effects of material characteristics on the structural characteristics and flavor substances retention of meat analogs. Food Hydrocoll. 2020, 105, 105752. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Neacsu, M.; McBey, D.; Johnstone, A.M. Meat Reduction and Plant-Based Food: Replacement of Meat: Nutritional, Health, and Social Aspects. In Sustainable Protein Sources; Nadathur, S., Wanasundara, J.P.D., Laurie Scanlin, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 359–375. ISBN 9780128027769. [Google Scholar]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Hear. Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Jakše, B.; Jakše, B.; Pinter, S.; Pajek, J.; Mis, N.F. Whole-Food Plant-Based Lifestyle Program and Decreased Obesity. Am. J. Lifestyle Med. 2020, 155982762094920. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC–Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33,883 meat-eaters and 31,546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy Protein: Impacts, Production, and Applications. In Sustainable Protein Sources; Nadathur, S., Wanasundara, J.P.D., Laurie Scanlin, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 23–45. ISBN 9780128027769. [Google Scholar]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Jones, O.G.; McClements, D.J. Functional Biopolymer Particles: Design, Fabrication, and Applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2016, 28, 308–318. [Google Scholar] [CrossRef]
- Berghout, J.; Pelgrom, P.; Schutyser, M.; Boom, R.; van der Goot, A. Sustainability assessment of oilseed fractionation processes: A case study on lupin seeds. J. Food Eng. 2015, 150, 117–124. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Davy, P.; Vuong, Q.V. Soy Milk By-product: Its Composition and Utilisation. Food Rev. Int. 2020, 1–23. [Google Scholar] [CrossRef]
- Jansens, K.J.; Rombouts, I.; Grootaert, C.; Brijs, K.; van Camp, J.; van der Meeren, P.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Rational Design of Amyloid-Like Fibrillary Structures for Tailoring Food Protein Techno-Functionality and Their Potential Health Implications. Compr. Rev. Food Sci. Food Saf. 2018, 18, 84–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. [Google Scholar] [CrossRef]
- Chen, H.; Shi, P.; Fan, F.; Chen, H.; Wu, C.; Xu, X.; Wang, Z.; Du, M. Hofmeister effect-assisted one step fabrication of fish gelatin hydrogels. LWT 2020, 121, 108973. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q.; Ding, Y. Physicochemical properties and microstructure of fish myofibrillar protein-lipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S. Impact of virgin coconut oil nanoemulsion on properties of croaker surimi gel. Food Hydrocoll. 2018, 82, 34–44. [Google Scholar] [CrossRef]
- Yang, R.; Xu, A.; Chen, Y.; Sun, N.; Zhang, J.; Jia, R.; Huang, T.; Yang, W. Effect of laver powder on textual, rheological properties and water distribution of squid (Dosidicus gigas) surimi gel. J. Texture Stud. 2020, 51, 968–978. [Google Scholar] [CrossRef]
- Huang, J.; Ye, B.; Wang, W.; Li, J.; Yi, S.; Li, X.; Xu, Y.; Mi, H. Incorporation effect of inulin and microbial transglutaminase on the gel properties of silver carp (Hypophthalmichthys molitrix) surimi. J. Food Meas. Charact. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Arêas, J.A.G. Extrusion of food proteins. Crit. Rev. Food Sci. Nutr. 1992, 32, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein–Protein Interactions during High-Moisture Extrusion for Fibrous Meat Analogues and Comparison of Protein Solubility Methods Using Different Solvent Systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dou, W.; Zhang, X.; Zhao, Y.; Zhang, Y.; Jiang, L.; Sui, X. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci. Technol. 2021, 109, 702–710. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Grahl, S.; Palanisamy, M.; Strack, M.; Meier-Dinkel, L.; Toepfl, S.; Mörlein, D. Towards more sustainable meat alternatives: How technical parameters affect the sensory properties of extrusion products derived from soy and algae. J. Clean. Prod. 2018, 198, 962–971. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Shah, F.; Xu, Y.; Wang, Q. High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: Effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocoll. 2020, 99, 105311. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Moreira, J.B.; Lim, L.-T.; Zavareze, E.D.R.; Dias, A.R.G.; Costa, J.A.V.; de Morais, M.G. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136. [Google Scholar] [CrossRef]
- Moreira, J.B.; Lim, L.-T.; Zavareze, E.D.R.; Dias, A.R.G.; Costa, J.A.V.; de Morais, M.G. Microalgae protein heating in acid/basic solution for nanofibers production by free surface electrospinning. J. Food Eng. 2018, 230, 49–54. [Google Scholar] [CrossRef]
- Mattice, K.D.; Marangoni, A.G. Comparing methods to produce fibrous material from zein. Food Res. Int. 2020, 128, 108804. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Xu, H.; Li, W.; Xu, L.; Yang, Y. Spinnability and rheological properties of globular soy protein solution. Food Hydrocoll. 2019, 90, 443–451. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhao, Y.; Yang, Y. Rheological properties of soy protein isolate solution for fibers and films. Food Hydrocoll. 2017, 64, 149–156. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhang, M.; Fang, Z. 3D printing of food: Pretreatment and post-treatment of materials. Crit. Rev. Food Sci. Nutr. 2019, 60, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Bhandari, B.; Prakash, S. 3D printing of meat. Meat Sci. 2019, 153, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D Printing in Food Processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Investigation on 3D printing ability of soybean protein isolate gels and correlations with their rheological and textural properties via LF-NMR spectroscopic characteristics. LWT 2020, 122, 109019. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]



| Product Type | Main Ingredients | Company |
|---|---|---|
| Tuna chunks, fish burgers, fish cakes and crab cakes | Six-legume blend (including peas, chickpeas, lentils, soy, fava beans and navy beans) | Good Catch |
| Fish filet and crab cakes | Soy, wheat, potato | Gardein |
| Caviar | Seaweeds | Plant-Based Foods |
| Fish fingers, tuna pate, fish cakes, smoked salmon | soy, potato, konjac, wheat | VBites |
| Ahimi®—raw tuna, Unami™—raw eel | Tomatoes/eggplants | Ocean Hugger Foods |
| Shrimp | Seaweeds | New Wave Shrimp |
| Product | Cooking Conditions | Hardness (N) | Chewiness (N) | Cohesiveness 2 | Springiness 2 | Resilience 2 | Reference |
|---|---|---|---|---|---|---|---|
| Pacu fillet | Grilled for 6 min, internal temperature of 60 °C | 4.91 ± 0.64 | 1.65 ± 0.25 | 0.46 ± 0.05 | NA | 0.17 ± 0.02 | [42] |
| Catfish fillet 1 | Baked at 149 °C, internal temperature of 74 °C | 2.16 ± 0.34 | 0.77 ± 0.18 | 0.47 ± 0.03 | 73.84 ± 2.65 | 23.5 ± 1.79 | [33] |
| Salmon fillet | Boiled in water for 5 min | 7.43 | 2.36 | 0.29 | 1.00 | NA | [43] |
| Sea Bass fillet | Smoked and dried for 3 h at 35 °C | 44 ± 3 | 21.2 ± 1.8 | 0.55 ± 0.01 | 0.59 ± 0.01 | NA | [44] |
| Surimi-based fish sausages 1 | Steamed at 90 °C for 30 min, internal temperature of 75 °C | 57.31 ± 0.06 | 5.59 ± 0.07 | 0.31 ± 0.00 | 0.32 ± 0.01 | NA | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazir, M.; Livney, Y.D. Plant-Based Seafood Analogs. Molecules 2021, 26, 1559. https://doi.org/10.3390/molecules26061559
Kazir M, Livney YD. Plant-Based Seafood Analogs. Molecules. 2021; 26(6):1559. https://doi.org/10.3390/molecules26061559
Chicago/Turabian StyleKazir, Meital, and Yoav D. Livney. 2021. "Plant-Based Seafood Analogs" Molecules 26, no. 6: 1559. https://doi.org/10.3390/molecules26061559
APA StyleKazir, M., & Livney, Y. D. (2021). Plant-Based Seafood Analogs. Molecules, 26(6), 1559. https://doi.org/10.3390/molecules26061559