Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies
Abstract
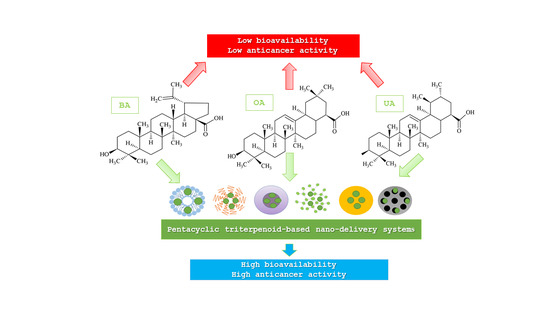
:1. Introduction
2. Biological Activities of Pentacyclic Triterpenoids
2.1. Lupane-Type Triterpenoids
2.2. Oleanane-Type Triterpenoids
2.3. Ursane-Type Triterpenoids
3. Drug Delivery Systems
3.1. Organic Nanocarriers
3.2. Inorganic Nanocarriers
3.3. Passive and Active Targeting
3.4. Challenges in Nanoformulations Design and Development
4. Nanoformulations with Pentacyclic Triterpenoids in Anticancer Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Jager, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Cappiello, F.; Loffredo, M.R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M.L.; Botta, B.; Ghirga, F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics (Basel) 2020, 9, 325. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Szakiel, A.; Paczkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef] [Green Version]
- Boryczka, S.; Bebenek, E.; Wietrzyk, J.; Kempinska, K.; Jastrzebska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Kaps, A.; Chodurek, E.; Orchel, A.; Jaworska-Kik, M.; Bebenek, E.; Boryczka, S.; Kasperczyk, J. Influence of 28-O-propynoylbetulin on proliferation and apoptosis of melanotic and amelanotic human melanoma cells. Postepy Hig. Med. Dosw. 2016, 70, 1404–1408. [Google Scholar] [CrossRef]
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of Oleanane and Ursane Triterpenic Acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef]
- Pattnaik, B.; Vadithe, L.N.; Sistla, R.; Uppuluri, V.M. Synthesis of ring-C modified oleanolic acid derivatives and their cytotoxic evaluation. Bioorg. Chem. 2016, 68, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, L.; Samec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.; Abhari, B.A.; Hinrichs, T.M.; Fulda, S.; Liese, J. Identification of a novel oxidative stress induced cell death by Sorafenib and oleanolic acid in human hepatocellular carcinoma cells. Biochem. Pharmacol. 2016, 118, 9–17. [Google Scholar] [CrossRef]
- Shi, Y.; Song, Q.; Hu, D.; Zhuang, X.; Yu, S.; Teng, D. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J. Physiol. Pharmacol. 2016, 20, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, L.; Skapska, S.; Marszalek, K. Ursolic Acid--A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [Green Version]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Liu, H.; Lv, H.; Zhang, J.; Zhou, J.; Zhao, Z. Preparation, characterization, and in vitro/vivo studies of oleanolic acid-loaded lactoferrin nanoparticles. Drug Des. Dev, Ther. 2017, 11, 1417–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple Strategies to Improve Oral Bioavailability by Fabricating Coamorphous Forms of Ursolic Acid with Piperine: Enhancing Water-Solubility, Permeability, and Inhibiting Cytochrome P450 Isozymes. Mol. Pharm. 2020, 17, 4443–4462. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Fang, Y.; Zhao, R.; Chen, F.; Yang, M.; Jiang, J.; Chen, Z.; Yuan, X.; Jia, L. Evolution from small molecule to nano-drug delivery systems: An emerging approach for cancer therapy of ursolic acid. Asian J. Pharm. Sci. 2020, 15, 685–700. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials (Basel) 2019, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliskan, Y.; Dalgic, A.D.; Gerekci, S.; Gulec, E.A.; Tezcaner, A.; Ozen, C.; Keskin, D. A new therapeutic combination for osteosarcoma: Gemcitabine and Clofazimine co-loaded liposomal formulation. Int. J. Pharm. 2019, 557, 97–104. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Witteloostuijn, S.B.; Pedersen, S.L.; Jensen, K.J. Half-Life Extension of Biopharmaceuticals using Chemical Methods: Alternatives to PEGylation. ChemMedChem 2016, 11, 2474–2495. [Google Scholar] [CrossRef] [PubMed]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers (Basel) 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 17 March 2021).
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hu, H.Z.; Qing, X.C.; Zhang, Z.C.; Shao, Z.W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef] [Green Version]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tsukigawa, K.; Fang, J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy--Problems, Solutions, and Prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef]
- Chen, S.; Yang, K.; Tuguntaev, R.G.; Mozhi, A.; Zhang, J.; Wang, P.C.; Liang, X.J. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine 2016, 12, 269–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Zhang, S.; Chen, Z.; Chen, A.T.; Ma, J.; Deng, G.; Xu, W.; Zhou, J.; Yu, Z.Q.; Yao, G.; et al. Synergistic Chemotherapy for Breast Cancer and Breast Cancer Brain Metastases via Paclitaxel-Loaded Oleanolic Acid Nanoparticles. Mol. Pharm. 2020, 17, 1343–1351. [Google Scholar] [CrossRef]
- Kahraman, E.; Gungor, S.; Ozsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, J.; Chen, Q. Synthesis, Characterization of Liposomes Modified with Biosurfactant MEL-A Loading Betulinic Acid and Its Anticancer Effect in HepG2 Cell. Molecules 2019, 24, 3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Tang, S.; Tong, Q. Oleanolic acid liposomes with polyethylene glycol modification: Promising antitumor drug delivery. Int. J. Nanomed. 2012, 7, 3517–3526. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhu, R.; Wang, M.; Xing, S.; Li, L.; He, Y.; Cao, W.; Gao, D. Targeted therapy of octreotide-modified oleanolic acid liposomes to somatostatin receptor overexpressing tumor cells. Nanomedicine (Lond) 2017, 12, 927–940. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Raza, S.M.; Bashir, S.; Madni, A.; Khan, M.W.; Ma, X.; Xiang, G. Liposomal co-delivered oleanolic acid attenuates doxorubicin-induced multi-organ toxicity in hepatocellular carcinoma. Oncotarget 2017, 8, 47136–47153. [Google Scholar] [CrossRef] [Green Version]
- Caldeira de Araujo Lopes, S.; Vinicius Melo Novais, M.; Salviano Teixeira, C.; Honorato-Sampaio, K.; Tadeu Pereira, M.; Ferreira, L.A.; Braga, F.C.; Cristina Oliveira, M. Preparation, physicochemical characterization, and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid. Biomed. Res. Int. 2013, 2013, 467147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, Y.; Gao, Z.; Gao, D.; Li, N.; Bian, Y.; Dai, K.; Liu, Z. Self-assembly and cytotoxicity study of PEG-modified ursolic acid liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C Mater. Biol. Appl 2017, 71, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yi, Y.; Liu, L.; Lin, Y.; Li, J.; Ruan, J.; Zhong, Z. Polymeric micelles loading with ursolic acid enhancing anti-tumor effect on hepatocellular carcinoma. J. Cancer 2019, 10, 5820–5831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, D.; Ding, J.; Xu, H.; Li, X.; Sun, W. Efficient delivery of ursolic acid by poly(N-vinylpyrrolidone)-block-poly (epsilon-caprolactone) nanoparticles for inhibiting the growth of hepatocellular carcinoma in vitro and in vivo. Int. J. Nanomed. 2015, 10, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Antonio, E.; Antunes, O.D.R.J.; de Araujo, I.S.; Khalil, N.M.; Mainardes, R.M. Poly(lactic acid) nanoparticles loaded with ursolic acid: Characterization and in vitro evaluation of radical scavenging activity and cytotoxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 156–166. [Google Scholar] [CrossRef]
- Baishya, R.; Nayak, D.K.; Kumar, D.; Sinha, S.; Gupta, A.; Ganguly, S.; Debnath, M.C. Ursolic Acid Loaded PLGA Nanoparticles: In vitro and in vivo Evaluation to Explore Tumor Targeting Ability on B16F10 Melanoma Cell Lines. Pharm. Res. 2016, 33, 2691–2703. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Dong, Y. Ursolic acid nanoparticles inhibit cervical cancer growth in vitro and in vivo via apoptosis induction. Int. J. Oncol. 2017, 50, 1330–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprean, C.; Zambori, C.; Borcan, F.; Soica, C.; Zupko, I.; Minorics, R.; Bojin, F.; Ambrus, R.; Muntean, D.; Danciu, C.; et al. Anti-proliferative and antibacterial in vitro evaluation of the polyurethane nanostructures incorporating pentacyclic triterpenes. Pharm. Biol. 2016, 54, 2714–2722. [Google Scholar] [CrossRef] [Green Version]
- Saneja, A.; Kumar, R.; Mintoo, M.J.; Dubey, R.D.; Sangwan, P.L.; Mondhe, D.M.; Panda, A.K.; Gupta, P.N. Gemcitabine and betulinic acid co-encapsulated PLGA-PEG polymer nanoparticles for improved efficacy of cancer chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 764–771. [Google Scholar] [CrossRef]
- Pandita, A.; Kumar, B.; Manvati, S.; Vaishnavi, S.; Singh, S.K.; Bamezai, R.N. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS ONE 2014, 9, e107154. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.B.M.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Xie, X.; Wang, C.; You, J.; Mo, F.; Jin, B.; Chen, J.; Shao, J.; Chen, H.; et al. Dendrimeric anticancer prodrugs for targeted delivery of ursolic acid to folate receptor-expressing cancer cells: Synthesis and biological evaluation. Eur. J. Pharm. Sci. 2015, 70, 55–63. [Google Scholar] [CrossRef]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in vitro and in vivo. Sci. Rep. 2016, 6, 30782. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, B.; Liu, Y.; Zheng, G.; Guo, Y.; Zhao, R.; Jiang, K.; Fan, L.; Shao, J. A self-assembly nanodrug delivery system based on amphiphilic low generations of PAMAM dendrimers-ursolic acid conjugate modified by lactobionic acid for HCC targeting therapy. Nanomedicine 2018, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, K.; Li, X.; Xiao, S.; Zheng, D.; Zhu, P.; Li, C.; Liu, J.; He, J.; Lei, J.; et al. A novel self-assembled nanoparticle platform based on pectin-eight-arm polyethylene glycol-drug conjugates for co-delivery of anticancer drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 28–41. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, K.F.; Li, C.X.; Wang, L.Y.; Liu, J.; He, J.; Lei, J.D.; Liu, X.Y. Self-assembled nanoparticles based on a carboxymethylcellulose-ursolic acid conjugate for anticancer combination therapy. RSC Adv. 2017, 7, 36256–36268. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Li, G.L.; Zhu, S.B.; Jing, F.C.; Liu, R.D.; Li, S.S.; He, J.; Lei, J.D. A Self-assembled Nanoparticle Platform Based on Amphiphilic Oleanolic Acid Polyprodrug for Cancer Therapy. Chin. J. Polym. Sci. 2020, 38, 819–829. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcas, C.; Mihali, C.V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Cui, R.; Xu, S.; Liu, Y. Synergism of cisplatin-oleanolic acid co-loaded hybrid nanoparticles on gastric carcinoma cells for enhanced apoptosis and reversed multidrug resistance. Drug Deliv. 2020, 27, 191–199. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Liu, Y.; Fan, L.; Lin, L.; Xu, Y.; Chen, S.; Shao, J. pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur. J. Pharm. Sci. 2017, 96, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.Y.; Xu, X.J.; Guan, S.Y.; Zhang, R.H.; Zhang, X.Y.; Kang, Y.; Wu, J. Nanodrug Carrier Based on Poly(Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Colombo, E.; Polito, L.; Biocotino, M.; Marzullo, P.; Hyeraci, M.; Via, L.D.; Passarella, D. New Class of Betulinic Acid-Based Nanoassemblies of Cabazitaxel, Podophyllotoxin, and Thiocolchicine. ACS Med. Chem. Lett. 2020, 11, 895–898. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [Green Version]
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, I.V.; Seibert, J.B.; Carneiro, S.P.; de Souza, G.H.B.; dos Santos, O.D.H.; Lopes, N.P. Preparation and in vitro Evaluation of alpha and beta-Amyrins Loaded Nanoemulsions. Curr. Pharm. Biotechnol. 2013, 14, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Junior, W.F.; Bezerra de Menezes, D.L.; de Oliveira, L.C.; Koester, L.S.; Oliveira de Almeida, P.D.; Lima, E.S.; de Azevedo, E.P.; da Veiga Junior, V.F.; Neves de Lima, A.A. Inclusion Complexes of beta and HPbeta-Cyclodextrin with alpha, beta Amyrin and In Vitro Anti-Inflammatory Activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]






| Pentacyclic Triterpenoid | DDSs | Cell Line | Animal Model | Effects | Reference |
|---|---|---|---|---|---|
| Betulin | Gold nanoparticles | A375, B164A5, 1BR3, and HaCaT | − | Increased cytotoxicity and induction of apoptosis. | [78] |
| Betulinic acid | Liposomes | HepG2 | − | Inhibition of cell cycle, increased stability, and induction of apoptosis. | [53] |
| Polymeric nanoparticles | PANC-1 | Ehrlich Ascites Carcinoma in Swiss albino male mice | Decreased cell proliferation, enhanced ROS production, induction of apoptosis, and reduced tumor volume. | [68] | |
| MDA-MB-231 and HEp-2 | − | Decreased cell proliferation and increased cytotoxicity. | [71] | ||
| Self-assembled nanoparticles | A2780 | − | Increased cytotoxicity and decreased cell proliferation. | [85] | |
| Polymer-drug conjugates | A2780 | − | Increased cytotoxicity and decreased cell proliferation. | [85] | |
| Oleanolic acid | Liposomes | HeLa | − | Increased cytotoxicity. | [54] |
| A549 | − | Increased cellular uptake and decreased cell proliferation. | [55] | ||
| HepG2, HepG3B, H9C2, and L-02 | HepG2 tumor-bearing female BALB/c mice and female Kunming mice | Increased anticancer activity and decreased doxorubicin (DOX) toxicity. | [56] | ||
| Polymeric nanoparticles | MCF-7, T47D, MDA-MB-231, and MDA-MB-361 | − | No significant effect on anticancer activity. | [67] | |
| − | Male Sprague Dawley rats | Improved oral absorption and bioavailability. | [25] | ||
| Hybrid nanoparticles | MGC-803 and NIT3T3 | MGC-803 tumor-bearing male BALB/c mice | Increased stability, biocompatibility and tumor-targeting, and induction of apoptosis. | [79] | |
| Self-assembled nanoparticles | 4T1 and MCF-7 | 4T1 tumor-bearing female BALB/c mice | Sustained drug release and increased cytotoxicity. | [77] | |
| MDA-MB-231-WT, MDA-MB-231-BR, MCF-7, and NHA | MDA-MB-231-WT tumor-bearing female athymic NCr-nu/nu mice, and MDA-MB-231-BR tumor-bearing female athymic NCr-nu/nu mice | Inhibition of cell cycle, improved paclitaxel (PTX) bioavailability, induction of autophagy and apoptosis, and inhibition of efflux transporters. | [48] | ||
| Ursolic acid | Liposomes | MDA-MB-231 and LNCaP | − | Improved stability and decreased cell proliferation. | [57] |
| EC-304 | − | Sustained drug release, decreased cell proliferation, and increased stability. | [58] | ||
| HeLa | U14 tumor-bearing female CD-1 mice | Increased tumor-targeting. | [59] | ||
| Polymeric micelles | HepG2 and L-02 | H22 tumor-bearing male Kunming mice | Decreased cell proliferation, decreased cell migration, and increased survival time. | [60] | |
| Polymeric nanoparticles | SGC7901 | − | Decreased cell proliferation, decreased cyclooxygenase 2 (COX-2) expression and increased caspase-3 activity. | [61] | |
| H22 | H22 tumor-bearing ICR mice | Decreased cell proliferation and increased stability. | [62] | ||
| B16F10 | − | Increased cytotoxicity and sustained drug release. | [63] | ||
| B16F10 | B16F10 tumor-bearing male BALB/c mice | Increased cytotoxicity, increased cellular uptake, and sustained drug release. | [64] | ||
| CaSki, HeLa, C4-1, SiHa, 293T, and L-02 | CaSki, HeLa, and SiHa tumor-bearing male athymic nude mice | Inhibition of cell cycle, cell migration, and invasion, as well as an induction of apoptosis. | [65] | ||
| HepG2, Caco-2, and Y-79 | − | Differentiated impact on cell proliferation and cytotoxicity. | [66] | ||
| MCF-7, T47D, MDA-MB-231, and MDA-MB-361 | − | No significant effect on anticancer activity. | [67] | ||
| MCF-7 and Colo205 | MCF-7 tumor-bearing female BALB/c mice | Increased tumor-targeting and cellular uptake. | [73] | ||
| Dendrimers | HepG2 and HeLa | − | Increased tumor-targeting and cellular uptake. | [72] | |
| SMMC7721 and HeLa | H22 tumor-bearing Sprague Dawley rats and H22 tumor-bearing Kunming mice | Increased cytotoxicity and tumor-targeting, decreased migration, adhesion, metastasis, and tumor growth. | [74] | ||
| Polymer-drug conjugates | 4T1 and MCF-7 | 4T1 tumor-bearing female BALB/c mice | Increased stability and survival rate. | [75] | |
| 4T1 | 4T1 tumor-bearing female BALB/c mice | Increased stability and survival rate, decreased tumor growth. | [76] | ||
| Mesoporous silica nanoparticles | HepG2 | − | Increased cytotoxicity. | [81] | |
| HeLa | H22 tumor-bearing nude mice | Decreased cell proliferation, invasion and metastasis, as well as the inhibition of the cell cycle. | [82] | ||
| SMMC7721, HepG2, Huh-7, and HeLa | H22 tumor-bearing nude Kunming mice | Increased cellular uptake and decreased metastasis. | [83] | ||
| Poly(ursolic acid) nanoparticles | CT26 and NIH 3T3 | CT26 tumor-bearing male Sprague Dawley rats and CT26 tumor-bearing male BALB/c mice | Increased stability, accumulation in cancer tissues, cytotoxicity and cellular uptake, and inhibition of the cell cycle and tumor progression. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules 2021, 26, 1764. https://doi.org/10.3390/molecules26061764
Kaps A, Gwiazdoń P, Chodurek E. Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules. 2021; 26(6):1764. https://doi.org/10.3390/molecules26061764
Chicago/Turabian StyleKaps, Anna, Paweł Gwiazdoń, and Ewa Chodurek. 2021. "Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies" Molecules 26, no. 6: 1764. https://doi.org/10.3390/molecules26061764
APA StyleKaps, A., Gwiazdoń, P., & Chodurek, E. (2021). Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules, 26(6), 1764. https://doi.org/10.3390/molecules26061764