Nanoproteomic Approach for Isolation and Identification of Potential Biomarkers in Human Urine from Adults with Normal Weight, Overweight and Obesity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of FCSNP
2.2. Evaluation of the FCSNP in the Capture of LMW Proteins and Exclusion of the High Molecular Weight (HMW) Proteins
2.3. Evaluation of the FCSNP in the Capture of Potential Biomarkers from Pooled Urine Samples
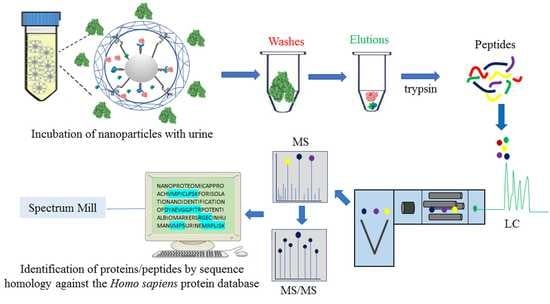
3. Subjects and Methods
3.1. Subjects
- Five adult women with normal weight (W-NW) and a mean age at recruitment of 35.2 ± 8.9 years (range 22–47) and a mean BMI of 22.6 ± 0.4.
- Five adult men with normal weight (M-NW) and a mean age at recruitment of 36.2 ± 11.8 years (range 24–52) and a mean BMI of 22.8 ± 1.1
- Five adult women with overweight (W-OW) and a mean age at recruitment of 36.8 ± 10.6 years (range 24–51) and a mean BMI of 27.5 ± 1.8.
- Five adult men with overweight (M-OW) and a mean age at recruitment of 31.6 ± 7.6 years (range 21–41) and a mean BMI of 29 ± 3.8
- Five adult women with obesity (W-OB) and a mean age at recruitment of 35.8 ± 8.3 years (range 28–46) and a mean BMI of 34.7 ± 2.4.
- Five adult men with obesity (M-OB) and a mean age at recruitment of 27.6 ± 2.9 years (range 24–32) and a mean BMI of 37.2 ± 4.3.
3.2. Sample Collection and Processing
3.3. Synthesis and Characterization of Core-Shell Silica Nanoparticles
3.4. Evaluation of the FCSNP in the Capture of LMW Proteins and Exclusion of HMW in Urine
3.5. Protein Digestion and Analysis by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) of Pooled Samples
3.6. Protein Identification and Differential Abundance Cluster Representation (Heat Map)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Merrell, K.; Southwick, K.; Graves, S.W.; Sean Esplin, M.; Lewis, N.E.; Thulin, C.D. Analysis of low-abundance, low-molecular-weight serum proteins using mass spectrometry. J. Biomol. Tech. 2004, 15, 238–248. [Google Scholar]
- Hernandez-Leon, S.; Sarabia-Sainz, J.; Montfort, G.; Guzman-Partida, A.; Robles-Burgueño, M.; Vazquez-Moreno, L. Novel Synthesis of Core-Shell Silica Nanoparticles for the Capture of Low Molecular Weight Proteins and Peptides. Molecules 2017, 22, 1712. [Google Scholar] [CrossRef] [Green Version]
- Brodkin, E.; Copes, R.; Mattman, A.; Kennedy, J.; Kling, R.; Yassi, A. Urine proteomics: The present and future of measuring urinary protein components in disease. CMJA 2007, 176, 59–63. [Google Scholar]
- Birn, H.; Christensen, E.I. Renal albumin absorption in physiology and pathology. Kidney Int. 2006, 69, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.E.; Sexton, W.; Benson, K.; Sutphen, R.; Koomen, J. Urine collection and processing for protein biomarker discovery and quantification. Cancer Epidemiol. Biomark. Prev. 2010, 19, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Candiano, G.; Santucci, L.; Petretto, A.; Bruschi, M.; Dimuccio, V.; Urbani, A.; Bagnasco, S.; Ghiggeri, G.M. 2D-electrophoresis and the urine proteome map: Where do we stand? J. Proteom. 2010, 73, 829–844. [Google Scholar] [CrossRef]
- Sǎrmǎşan, C.; Drǎghici, S.; Daina, L. Identification, communication and management of risks relating to drinking water pollution in Bihor County. Environ. Eng. Manag. J. 2008, 7, 769–774. [Google Scholar] [CrossRef]
- Yusa, V.; Ye, X.; Calafat, A.M. Methods for the determination of biomarkers of exposure to emerging pollutants in human specimens. TrAC Trends Anal. Chem. 2012, 38, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Ni, J.; Beretov, J.; Cozzi, P.; Willcox, M.; Wasinger, V.; Walsh, B.; Graham, P.; Li, Y. Urinary biomarkers in prostate cancer detection and monitoring progression. Crit. Rev. Oncol. Hematol. 2017, 118, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Luchini, A.; Fredolini, C.; Espina, B.H.; Meani, F.; Reeder, A.; Rucker, A.; Petricoin, E.F., III; Liotta, L.A. Nanoparticle Technology: Addressing the fundamental roadblocks to protein biomarker discovery. Curr. Mol. Med. 2010, 10, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, C.; Patanarut, A.; George, T.; Bishop, B.; Zhou, W.; Fredolini, C.; Ross, M.M.; Espina, V.; Pellacani, G.; Petricoin, E.F.; et al. Core-shell hydrogel particles harvest, concentrate and preserve labile low abundance biomarkers. PLoS ONE 2009, 4, e4763. [Google Scholar] [CrossRef]
- Tamburro, D.; Fredolini, C.; Espina, V.; Douglas, T.A.; Ranganathan, A.; Ilag, L.; Zhou, W.; Russo, P.; Espina, B.H.; Muto, G.; et al. Multifunctional core-shell nanoparticles: Discovery of previously invisible biomarkers. J. Am. Chem. Soc. 2011, 133, 19178–19188. [Google Scholar] [CrossRef] [PubMed]
- Fredolini, C.; Meani, F.; Luchini, A.; Zhou, W.; Russo, P.; Ross, M.; Patanarut, A.; Tamburro, D.; Gambara, G.; Ornstein, D.; et al. Investigation of the ovarian and prostate cancer peptidome for candidate early detection markers using a novel nanoparticle biomarker capture technology. AAPS J. 2010, 12, 504–518. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Lu, Y.; Shao, J.; Liang, X.J.; Xu, Y. Nanoproteomics: A new sprout from emerging links between nanotechnology and proteomics. Trends Biotechnol. 2013, 31, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Balashanmugam, P.; Mukeshkumar, D.J.; Kalaichelvan, P.T. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016, 182, 8–20. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Shono, M.; Ishimura, K. Synthesis, characterization, and biological applications of multifluorescent silica nanoparticles. Anal. Chem. 2007, 79, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Jackson, J.B.; Halas, N.J.; Lee, T.R. Preparation and Characterization of Gold Nanoshells Coated with Self-Assembled Monolayers. Langmuir 2002, 18, 4915–4920. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Leon, S.G.; Sarabia-Sainz, J.A.; Ramos-Clamont Montfort, G.; Huerta-Ocampo, J.Á.; Guzman-Partida, A.M.; Robles-Burgueño, M.d.R.; Burgara-Estrella, A.J.; Vazquez-Moreno, L. Bifunctional nickel-iminodiacetic acid-core-shell silica nanoparticles for the exclusion of high molecular weight proteins and purification of His-tagged recombinant proteins. RSC Adv. 2019, 9, 11038–11045. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hwang, Y.; Kim, K.-N.; Ahn, C.; Sung, H.K.; Ko, K.-P.; Oh, K.-H.; Ahn, C.; Park, Y.J.; Kim, S.; et al. Associations of urinary sodium levels with overweight and central obesity in a population with a sodium intake. BMC Nutr. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Hall, J.E.; Kuo, J.J.; Da Silva, A.A.; De Paula, R.B.; Liu, J.; Tallam, L. Obesity-associated hypertension and kidney disease. Curr. Opin. Nephrol. Hypertens. 2003, 12, 195–200. [Google Scholar] [CrossRef]
- Kumar, P.; Nandi, S.; Tan, T.Z.; Ler, S.G.; Chia, K.S.; Lim, W.Y.; Bütow, Z.; Vordos, D.; De laTaille, A.; Al-Haddawi, M.; et al. Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma. Oncotarget 2015, 6, 13539–13549. [Google Scholar] [CrossRef]
- Kirschenbaum, A.; Izadmehr, S.; Yao, S.; O’Connor-Chapman, K.L.; Huang, A.; Gregoriades, E.M.; Yakar, S.; Levine, A.C. Prostatic acid phosphatase alters the RANKL/OPG system and induces osteoblastic prostate cancer bone metastases. Endocrinology 2016, 157, 4526–4533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokugawa, Y.; Kunishige, I.; Kubota, Y.; Shimoya, K.; Nobunaga, T.; Kimura, T.; Saji, F.; Murata, Y.; Eguchi, N.; Oda, H.; et al. Lipocalin-Type Prostaglandin D Synthase in Human Male Reproductive Organs and Seminal Plasma1. Biol. Reprod. 1998, 58, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Anheuser, S.; Breiden, B.; Schwarzmann, G.; Sandhoff, K. Membrane lipids regulate ganglioside GM2 catabolism and GM2 activator protein activity. J. Lipid Res. 2015, 56, 1747–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefranc, M.P. Immunoglobulin and T cell receptor genes: IMGT® and the birth and rise of immunoinformatics. Front. Immunol. 2014, 5, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Alves, G.; Pereira, D.A.; Sandim, V.; Ornellas, A.A.; Escher, N.; Melle, C.; von Eggeling, F. Urine screening by Seldi-Tof, followed by biomarker identification, in a Brazilian cohort of patients with renal cell carcinoma (RCC). Int. Braz. J. Urol. 2013, 39, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Kohler, M.; Schänzer, W.; Thevis, M. Effects of Excercise on the Urinary Proteome. In Urine Proteomics in Kidney Disease Biomarker Discovery; Gao, Y., Ed.; Springer: Dordrecth, The Netherlands, 2015; Volume 845, pp. 121–131. [Google Scholar]
- Leendertz, S.A.J.; Metzger, S.; Skjerve, E.; Deschner, T.; Boesch, C.; Riedel, J.; Leendertz, F.H. A longitudinal study of urinary dipstick parameters in wild chimpanzees (Pan troglodytes verus) in Côte d’ivoire. Am. J. Primatol. 2010, 72, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Dessing, M.C.; Butter, L.M.; Teske, G.J.; Claessen, N.; Van der Loos, C.M.; Vogl, T.; Roth, J.; Van der Poll, T.; Florquin, S.; Leemans, J.C. S100A8/A9 is not involved in host defense against murine urinary tract infection. PLoS ONE 2010, 5, e13394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaja, C.A.; Scholes, D.; Hooton, T.M.; Stamm, W.E. Population-Based Epidemiologic Analysis of Acute Pyelonephritis. Clin. Infect. Dis. 2007, 45, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Ramos-Barron, A.; Ruiz-Criado, J.; Maroto, A.S.; Ortiz, A.; Gomez-Alamillo, C.; Arias, M.; Vivanco, F.; Alvarez-Llamas, G. Urinary Kininogen-1 and Retinol binding protein-4 respond to Acute Kidney Injury: Predictors of patient prognosis? Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Wada, J.; Eguchi, J.; Nakatsuka, A.; Teshigawara, S.; Murakami, K.; Ogawa, D.; Terami, T.; Katayama, A.; Tone, A.; et al. Urinary Fetuin-A Is a Novel Marker for Diabetic Nephropathy in Type 2 Diabetes Identified by Lectin Microarray. PLoS ONE 2013, 8, e77118. [Google Scholar] [CrossRef] [Green Version]
- Atmani, F.; Khan, S.R. Characterization of uronic-acid-rich inhibitor of calcium oxalate crystallization isolated from rat urine. Urol. Res. 1995, 23, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Good, D.M.; Zürbig, P.; Argilés, À.; Bauer, H.W.; Behrens, G.; Coon, J.J.; Dakna, M.; Decramer, S.; Delles, C.; Dominiczak, A.F.; et al. Naturally Occurring Human Urinary Peptides for Use in Diagnosis of Chronic Kidney Disease. Mol. Cell. Proteom. 2010, 9, 2424–2437. [Google Scholar] [CrossRef] [Green Version]
- Kentsis, A.; Ahmed, S.; Kurek, K.; Brennan, E.; Bradwin, G.; Steen, H.; Bachur, R. Detection and diagnostic value of urine leucine-rich α-2-glycoprotein in children with suspected acute appendicitis. Ann. Emerg. Med. 2012, 60, 78–83.e1. [Google Scholar] [CrossRef] [Green Version]
- Muffat, J.; Walker, D.W. Apolipoprotein D: An overview of its role in aging and age-related diseases. Cell Cycle 2010, 9, 269–273. [Google Scholar] [CrossRef] [Green Version]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2012, 12, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, H.; Yoshimura, A.; Inui, K.; Ideura, T.; Watanabe, H.; Wang, L.; Soininen, R.; Tryggvason, K. Heparan sulfate of perlecan is involved in glomerular filtration. J. Am. Soc. Nephrol. 2005, 16, 1703–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayapalan, J.J.; Ng, K.L.; Shuib, A.S.; Razack, A.H.A.; Hashim, O.H. Urine of patients with early prostate cancer contains lower levels of light chain fragments of inter-alpha-trypsin inhibitor and saposin B but increased expression of an inter-alpha-trypsin inhibitor heavy chain 4 fragment. Electrophoresis 2013, 34, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Thiel, S.; Jensenius, J.C.; Terai, I.; Fujita, T. Proteolytic Activities of Two Types of Mannose-Binding Lectin-Associated Serine Protease. J. Immunol. 2000, 165, 2637–2642. [Google Scholar] [CrossRef] [Green Version]
- Rampoldi, L.; Scolari, F.; Amoroso, A.; Ghiggeri, G.; Devuyst, O. The rediscovery of uromodulin (Tamm-Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011, 80, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Vasudev, N.S.; Banks, R.E. Biomarkers of Renal Cancer, 1st ed.; Elsevier Inc.: London, UK, 2016; ISBN 9780128030141. [Google Scholar]
- Kokichi, S.; Etsuko, O.; Nobuo, S.; Kazuhiko, T.; Tomoichi, K. Urinary α-glucosidase analysis for the detection of the adult form of Pompe’s disease. Clin. Chim. Acta 1977, 77, 61–67. [Google Scholar] [CrossRef]
- Mahyar, A.; Ayazi, P.; Gholmohammadi, P.; Moshiri, S.A.; Oveisi, S.; Esmaeily, S. The role of overweight and obesity in urinary tract infection in children. Infez. Med. 2016, 24, 38–42. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Morales-Amparano, M.B.; Ramos-Clamont Montfort, G.; Baqueiro-Peña, I.; del Refugio Robles-Burgueño, M.; Vázquez-Moreno, L.; Huerta-Ocampo, J.Á. Proteomic response of Saccharomyces boulardii to simulated gastrointestinal conditions and encapsulation. Food Sci. Biotechnol. 2019, 28, 831–840. [Google Scholar] [CrossRef] [PubMed]




| Protein | Accession Number | PMM (Da) a | Score | PM/Sc % b | W-NW c | Fold Change | M-NW f | Fold Change i | ||
|---|---|---|---|---|---|---|---|---|---|---|
| W-OW d | W-OB e | M-OW g | M-OB h | |||||||
| Cluster 1. Protein present/absent in either men or women. | ||||||||||
| Prostatic acid phosphatase | P15309 | 44,907.6 | 33.62 | 2/6.9 | X | X | X | √ | 0.19 | X |
| Immunoglobulin kappa variable 3D-20 | A0A0C4DH25 | 12,628.9 | 28.35 | 2/21.5 | X | X | X | √ | 0.27 | X |
| Semenogelin-2 | Q02383 | 65,557.4 | 29.46 | 2/4.8 | X | X | X | √ | 0.90 | 1.68 |
| Ganglioside GM2 activator | P17900 | 21,294.4 | 27.96 | 2/15.5 | X | X | X | X | √ | √ |
| Prostaglandin-H2 D-isomerase | P41222 | 21,256.7 | 25.74 | 2/17.3 | X | X | X | √ | 0.83 | X |
| Vitelline membrane outer layer protein 1 homolog | Q7Z5L0 | 22,047.2 | 49.96 | 4/33.6 | X | √ | √ | X | X | X |
| Protein S100-A8 | P05109 | 10,891.4 | 46.88 | 3/31.1 | √ | 0.27 | 0.18 | X | X | X |
| Protein S100-A9 | P06702 | 13,298.8 | 44.4 | 3/31.5 | √ | 0.08 | 0.57 | X | X | X |
| Hemoglobin subunit alpha | P69905 | 15,314.3 | 42.55 | 3/21.1 | √ | X | √ | X | X | X |
| Cluster 2. Protein abundance decreases as the body weight increases. | ||||||||||
| Kininogen-1 | P01042 | 73,040.1 | 116.52 | 9/16.3 | √ | 0.68 | X | √ | 0.34 | 0.03 |
| Alpha-2-HS-glycoprotein | P02765 | 40,122.7 | 76.81 | 5/20.7 | √ | 0.85 | X | √ | 0.95 | 0.42 |
| Protein AMBP | P02760 | 39,911.7 | 52.14 | 3/10.5 | √ | 0.93 | 0.17 | √ | 0.64 | 0.43 |
| Polymeric immunoglobulin receptor | P01833 | 84,480.2 | 51.28 | 3/6.5 | √ | 0.30 | X | √ | 0.29 | X |
| Cluster 3. Protein abundance increases as the body weight increases. | ||||||||||
| Serum albumin | P02768 | 71,362.3 | 84.63 | 6/9.8 | √ | 6.79 | 22.23 | X | √ | √ |
| Cluster 4. Protein abundance increases in overweight (OW) and decreases in obesity (OB). | ||||||||||
| Alpha-1-antitrypsin | P01009 | 46,906.8 | 120.34 | 7/22 | √ | 1.32 | 1.03 | √ | 1.64 | 1.26 |
| Leucine-rich alpha-2-glycoprotein | P02750 | 38,405.4 | 68.27 | 5/24.4 | X | √ | X | √ | 12.64 | 5.54 |
| Apolipoprotein D | P05090 | 21,560.4 | 67.97 | 5/24.3 | √ | 2.12 | 0.43 | √ | 3.42 | 0.60 |
| Beta-2-microglobulin | P61769 | 13,828.4 | 49.73 | 3/35.2 | √ | 1.38 | 1.20 | √ | 1.31 | 0.78 |
| Osteopontin | P10451 | 35,593.3 | 42.73 | 3/14.9 | √ | 1.35 | 0.60 | √ | 2.19 | 0.35 |
| Transthyretin | P02766 | 16,000.8 | 31.48 | 2/244 | X | √ | √ | X | √ | √ |
| Dystroglycan | Q14118 | 97,782 | 27.58 | 2/2.6 | √ | 1.44 | 1.33 | √ | 2.71 | 1.65 |
| Vesicular integral-membrane protein VIP36 | Q12907 | 40,570.3 | 88.09 | 6/18.8 | √ | 1.89 | 1.33 | √ | 1.44 | 1.00 |
| Cluster 5. Protein abundance increases in W-OW and decreases in W-OB. Decreases as body weight increases in men. | ||||||||||
| Immunoglobulin heavy constant gamma 4 | P01861 | 36,453.4 | 69.58 | 5/22.6 | √ | 1.46 | 0.29 | √ | 0.68 | 0.63 |
| Immunoglobulin heavy constant gamma 2 | P01859 | 36,527.6 | 47.65 | 4/15.9 | √ | 2.11 | 0.42 | √ | 0.73 | 0.72 |
| Immunoglobulin kappa variable 3–20 | P01619 | 12,671 | 30.84 | 2/21.5 | √ | 1.66 | X | √ | 0.56 | 0.38 |
| Cluster 6. Protein abundance increases in W-OW and decreases in W-OB. Increases as body weight increases in men. | ||||||||||
| Immunoglobulin lambda constant 2 | P0DOY2 | 11,464.5 | 72.9 | 5/74.5 | √ | 1.88 | 1.65 | √ | 0.71 | 1.68 |
| Basement membrane-specific heparan sulphate proteoglycan core protein | P98160 | 479,547.8 | 70.1 | 5/1.6 | √ | 1.06 | 0.35 | √ | 0.59 | 0.65 |
| Inter-alpha-trypsin inhibitor heavy chain H4 | Q14624 | 103,583.8 | 49.44 | 3/6.6 | √ | 6.05 | 0.22 | √ | 0.04 | 0.13 |
| Cluster 7. Proteins with heterogeneous abundance patterns. | ||||||||||
| Mannan-binding lectin serine protease 2 | O00187 | 77,241.4 | 107.62 | 710.4 | √ | 0.23 | 0.06 | √ | 0.43 | 1.02 |
| Hemoglobin subunit beta | P68871 | 16,112.2 | 115.05 | 6/46.9 | √ | 0.09 | 2.34 | √ | 0.24 | 0.51 |
| Immunoglobulin kappa constant | P01834 | 11,936 | 95.38 | 679.4 | √ | 0.96 | 1.12 | √ | 0.92 | 0.77 |
| Immunoglobulin heavy constant gamma 1 | P01857 | 36,618.7 | 85.98 | 6/26.9 | √ | 1.50 | 0.72 | √ | 1.05 | 1.24 |
| Uromodulin | P07911 | 72,498.3 | 74.37 | 6/9 | √ | 1.02 | 2.07 | √ | 1.55 | 1.00 |
| Prosaposin | P07602 | 59,937.4 | 35.85 | 2/4.7 | √ | 4.30 | 5.47 | √ | 0.80 | 0.62 |
| Cathepsin D | P07339 | 45,064.9 | 29.75 | 2/5.5 | √ | 0.51 | X | √ | 0.07 | 0.31 |
| Lysosomal alpha-glucosidase | P10253 | 106,177.6 | 31.4 | 2/3.5 | √ | 3.18 | 2.69 | √ | 1.17 | 2.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Leon, S.G.; Sarabia Sainz, J.A.-i.; Ramos-Clamont Montfort, G.; Huerta-Ocampo, J.Á.; Ballesteros, M.N.; Guzman-Partida, A.M.; Robles-Burgueño, M.d.R.; Vazquez-Moreno, L. Nanoproteomic Approach for Isolation and Identification of Potential Biomarkers in Human Urine from Adults with Normal Weight, Overweight and Obesity. Molecules 2021, 26, 1803. https://doi.org/10.3390/molecules26061803
Hernandez-Leon SG, Sarabia Sainz JA-i, Ramos-Clamont Montfort G, Huerta-Ocampo JÁ, Ballesteros MN, Guzman-Partida AM, Robles-Burgueño MdR, Vazquez-Moreno L. Nanoproteomic Approach for Isolation and Identification of Potential Biomarkers in Human Urine from Adults with Normal Weight, Overweight and Obesity. Molecules. 2021; 26(6):1803. https://doi.org/10.3390/molecules26061803
Chicago/Turabian StyleHernandez-Leon, Sergio G., Jose Andre-i Sarabia Sainz, Gabriela Ramos-Clamont Montfort, José Ángel Huerta-Ocampo, Martha Nydia Ballesteros, Ana M. Guzman-Partida, María del Refugio Robles-Burgueño, and Luz Vazquez-Moreno. 2021. "Nanoproteomic Approach for Isolation and Identification of Potential Biomarkers in Human Urine from Adults with Normal Weight, Overweight and Obesity" Molecules 26, no. 6: 1803. https://doi.org/10.3390/molecules26061803
APA StyleHernandez-Leon, S. G., Sarabia Sainz, J. A. -i., Ramos-Clamont Montfort, G., Huerta-Ocampo, J. Á., Ballesteros, M. N., Guzman-Partida, A. M., Robles-Burgueño, M. d. R., & Vazquez-Moreno, L. (2021). Nanoproteomic Approach for Isolation and Identification of Potential Biomarkers in Human Urine from Adults with Normal Weight, Overweight and Obesity. Molecules, 26(6), 1803. https://doi.org/10.3390/molecules26061803