Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of PCCF
2.1.1. Thickness
2.1.2. Film Color and Optical Characterization
2.1.3. Moisture Content and Water Solubility
2.1.4. Mechanical Properties
2.2. Structure Characterization of PCCF
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. X-ray Diffractometry (XRD)
2.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.4. Scanning Electron Microscope (SEM)
2.3. Antioxidant Activity
2.4. Antiseptic Capacity in Food System
3. Materials and Methods
3.1. Materials and Reagents
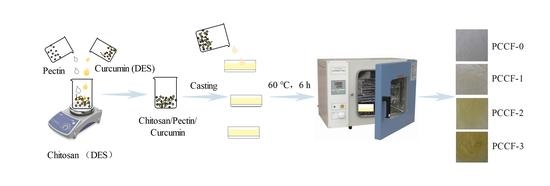
3.2. Preparation of PCCF
3.3. Characterization of PCCF
3.3.1. Thickness
3.3.2. Film Color and Optical Characterization
3.3.3. Determination of Moisture Content and Water Solubility
3.3.4. Mechanical Properties
3.4. Differential Scanning Calorimetry (DSC)
3.5. X-ray Diffractometry (XRD)
3.6. Fourier Transform Infrared Spectroscopy (FT-IR)
3.7. Scanning Electron Microscope (SEM)
3.8. Antioxidant Activity
3.9. Antiseptic Capacity in Food System
3.10. Statistic Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- The State of Food Security and Nutrition in the World 2020. Available online: http://www.fao.org/3/ca9692zh/online/ca9692zh.html#chapter-1_1 (accessed on 6 April 2021).
- Munesue, Y.; Masui, T.; Fushima, T. The effects of reducing food losses and food waste on global food insecurity, natural resources, and greenhouse gas emissions. Environ. Econ. Policy Stud. 2015, 17, 43–77. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Caleb, O.J.; Ngcobo, M.E.K. Effects of packaging and duration on quality of minimally processed and unpitted litchi cv. “Mauritius“ under low storage temperature. Heliyon 2020, 6, e03229. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.L.; Tran, D.H.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Effect of controlled atmosphere storage on the quality attributes and volatile organic compounds profile of dragon fruit (Hylocereus undatus). Postharvest Biol. Technol. 2020, 173, 111406. [Google Scholar] [CrossRef]
- Mulmule, M.D.; Shimmy, M.S.; Bambole, V.; Jamdar, S.N.; Rawat, K.P.; Sarma, K.S.S. Combination of electron beam irradiation and thermal treatment to enhance the shelf-life of traditional Indian fermented food (Idli). Radiat. Phys. Chem. 2017, 131, 95–99. [Google Scholar] [CrossRef]
- Herrero, L.; Quintanilla-López, J.E.; Fernández, M.A.; Gómara, B. Plasticisers and preservatives in commercial milk products: A comprehensive study on packages used in the Spanish market. Food Chem. 2020, 338, 128031. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Ríos, E.M. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2016, 68, 136–148. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef] [Green Version]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Nikolaeva, A.L.; Vorobiov, V.K.; Bobrova, N.V.; Abalov, I.V.; Smirnov, A.V.; Sokolova, M.P. Ionic conductivity and structure of chitosan films modified with lactic acid-choline chloride NADES. Polymers 2020, 12, 350. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.D.; Duri, S.; Harkins, A.L. Recyclable synthesis, characterization, and antimicrobial activity of chitosan-based polysaccharide composite materials. J. Biomed. Mater. Res. Part A 2013, 101, 2248–2257. [Google Scholar] [CrossRef] [Green Version]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep eutectic solvents and nonconventional technologies for blueberry-peel extraction: Kinetics, anthocyanin stability, and antiproliferative activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Piemontese, L.; Sergio, R.; Rinaldo, F.; Brunetti, L.; Perna, M.F.; Santos, M.A.; Capriati, V. Deep eutectic solvents as effective reaction media for the synthesis of 2-hydroxyphenylbenzimidazole-based scaffolds en route to donepezil-like compounds. Molecules 2020, 25, 574. [Google Scholar] [CrossRef] [Green Version]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Simultaneous processing of thermoplastic starch/wood biocomposite and in-situ fiber modification with deep eutectic solvent. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Silva, N.H.; Pinto, R.J.; Freire, C.S.; Marrucho, I.M. Production of lysozyme nanofibers using deep eutectic solvent aqueous solutions. Colloids Surf. B Biointerfaces 2016, 147, 36–44. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Song, S.T. Chitosan-gelatin-pectin composite films from polyion-complex hydrogels. Asian J. Chem. 2013, 25, 5363–5366. [Google Scholar] [CrossRef]
- Martínez, M.C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez, F.F.; Márquez Ríos, E. Hybrid nanofilms as topical anesthetics for pain-free procedures in dentistry. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Wei-Cai Zeng, W.-C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application—ScienceDirect. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Sueth-Santiago, V.; Mendes-Silva, G.P.; Decote-Ricardo, D.; Lima, M.E.F. Curcumin, the golden powder from turmeric: Insights into chemical and biological activities. Química Nova 2015, 38, 538–552. [Google Scholar]
- Bessone, F.; Argenziano, M.; Grillo, G.; Ferrara, B.; Pizzimenti, S.; Barrera, G.; Cravotto, G.; Guiot, C.; Stura, I.; Cavalli, R.; et al. Low-dose curcuminoid-loaded in dextran nanobubbles can prevent metastatic spreading in prostate cancer cells. Nanotechnology 2019, 30, 214004. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- Susan, H.; Douglas, K. Curcumin: A review of its′ effects on human health. Foods 2017, 6, 92. [Google Scholar]
- Gopinath, D.; Ahmed, M.; Gomathi, K.; Chitra, K.; Sehgal, P.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2016, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Varaprasad, K.; Reddy, G.V.S.; Rajulu, A.V.; Zhang, J. Preparation and characterization of cellulose/curcumin composite films. RSC Adv. 2012, 2, 8483–8488. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sullca, C.; Atarés, L.; Vargas, M.; Chiralt, A. Physical and antimicrobial properties of compression-molded cassava starch-chitosan films for meat preservation. Food Bioprocess Technol. 2018, 11, 1339–1349. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of Pectin as a green polymer electrolyte on the transport properties of Chitosan-Pectin membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [CrossRef] [Green Version]
- Anter, H.M.; Hashim, I.I.A.; Awadin, W.; Meshali, M.M. Novel anti-inflammatory film as a delivery system for the external medication with bioactive phytochemical “Apocynin”. Drug Des. Dev. Ther. 2018, 12, 2981. [Google Scholar] [CrossRef] [Green Version]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 315–322. [Google Scholar] [CrossRef]
- Chunhua Wu, C.; Sun, J.; Chen, M.; Ge, Y.; Ma, J.; Hu, Y.; Pang, J.; Yan, Z. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring. Food Hydrocoll. 2019, 95, 308–317. [Google Scholar]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The influence of Aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef]
- Xin, Y.; Bligh, M.W.; Kinsela, A.S.; Wang, Y.; Waite, T.D. Calcium-mediated polysaccharide gel formation and breakage: Impact on membrane foulant hydraulic properties. J. Membr. Sci. 2015, 475, 395–405. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Particle formation pathways and polymorphism of curcumin induced by ultrasound and additives during liquid antisolvent precipitation. CrystEngComm 2014, 16, 11102–11114. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Almeida, C.M.; Magalhães, J.M.; Souza, H.K.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent (DES) and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Almeida, C.M.; Magalhães, J.M.; Souza, H.K.; Gonçalves, M.P. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar]








| Sample | Thickness (mm) | Transparency (%) | L* | a* | b* |
|---|---|---|---|---|---|
| PCCF-0 | 0.186 ± 0.019 d | 30.27 ± 2.87 a | 49.64 ± 0.96 d | 4.31 ± 0.41 a | 30.365 ± 0.47 d |
| PCCF-1 | 0.328 ± 0.035 c | 18.08 ± 3.02 b | 52.78 ± 0.66 c | 2.26 ± 0.29 b | 38.17 ± 1.3 c |
| PCCF-2 | 0.420 ± 0.035 b | 9.22 ± 0.01 c | 54.98 ± 1.16 b | 1.90 ± 0.31 b | 38.39 ± 0.63 b |
| PCCF-3 | 0.426 ± 0.025 a | 7.72 ± 0.81 d | 57.82 ± 0.07 a | 0.56 ± 0.07 b | 44.86 ± 0.44 a |
| Sample | IR Assignment | ||||
|---|---|---|---|---|---|
| NR2, –NH, O–H Stretching | C–H Stretching | C=O Stretching | C=C Stretching, N–H Bending Stretching | C=C Stretching, C–OH Stretching | |
| PCCF0 | 3381 | 2887 | 1735 | 1595 | 1479 |
| PCCF1 | 3448, 3419, 3409, 3388 | 2935 | 1733 | 1595 | 1479 |
| PCCF2 | 3411, 3382 | 2935 | 1733 | 1595 | 1481 |
| PCCF3 | 3413, 3379 | 2935 | 1733 | 1593 | 1481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Zheng, X.; Li, L.; Ma, L.; Zhao, Q.; Chang, S.; You, L. Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films. Molecules 2021, 26, 2152. https://doi.org/10.3390/molecules26082152
Xie Q, Zheng X, Li L, Ma L, Zhao Q, Chang S, You L. Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films. Molecules. 2021; 26(8):2152. https://doi.org/10.3390/molecules26082152
Chicago/Turabian StyleXie, Qingtong, Xudong Zheng, Liuting Li, Liqun Ma, Qihui Zhao, Shiyuan Chang, and Lijun You. 2021. "Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films" Molecules 26, no. 8: 2152. https://doi.org/10.3390/molecules26082152
APA StyleXie, Q., Zheng, X., Li, L., Ma, L., Zhao, Q., Chang, S., & You, L. (2021). Effect of Curcumin Addition on the Properties of Biodegradable Pectin/Chitosan Films. Molecules, 26(8), 2152. https://doi.org/10.3390/molecules26082152