Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells
Abstract
:1. Introduction
2. Results
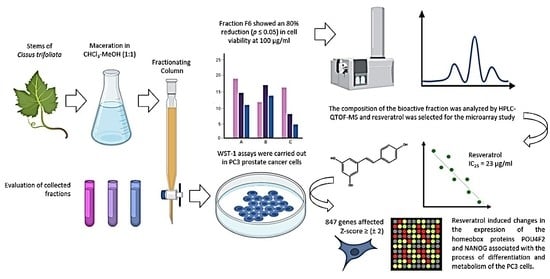
2.1. Cytotoxicity of the Fractions from the CHCl3-MeOH Extract
2.2. Chemical Analysis of the Active Fraction F6
2.3. Growth Inhibition of PC3 Cells by Resveratrol
2.4. Transcriptional Effects of Resveratrol on PC3 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Column Chromatography
4.3. Cell Culture and Cytotoxic Assay
4.4. HPLC-QTOF-MS Analysis
4.5. Determination of the IC50 of Resveratrol
4.6. Microarrays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crawford, E.D.; Andriole, G.L.; Marberger, M.; Rittmaster, R.S. Reduction in the risk of prostate cancer: Future directions after the Prostate Cancer Prevention Trial. Urology 2010, 75, 502–509. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ahmad, A.; Kong, D.; Bao, B. Recent progress on nutraceutical research in prostate cancer. Cancer Metastasis Rev. 2014, 33, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.; Patil, M. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013, 4, 338–346. [Google Scholar] [CrossRef]
- Méndez-López, L.F.; Garza-González, E.; Ríos, M.Y.; Ramírez-Cisneros, M.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.D.R. Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. Int. J. Mol. Sci. 2020, 21, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magana, A.; Gama Campillo, L.M.; Mariaca Méndez, R. El uso de las plantas medicinales en las comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–262. [Google Scholar]
- Quiros-Moran, D. Guide to Afro-Cuban Herbalism; Author House: Bloomington, IN, USA, 2009. [Google Scholar]
- Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. 2000, 14, 479–488. [Google Scholar] [CrossRef]
- Kong, C.S.; Jeong, C.H.; Choi, J.S.; Kim, K.J.; Jeong, J.W. Antiangiogenic effects of p-coumaric acid in human endothelial cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B.J. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, L.M.; Zigrossi, D.A.; Tauber, R.A.; Hightower, C.; Milner, J.A. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr. Cancer. 2000, 38, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G.J. Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-Y.; Chen, P.-N.; Hong, M.-C.; Hseu, Y.-C.; Chen, K.-M.; Hsu, L.-S.; Chen, W. Naringenin Attenuated Prostate Cancer Invasion via Reversal of Epithelial-to-Mesenchymal Transition and Inhibited uPA Activity. Anticancer Res. 2018, 38, 6753–6758. [Google Scholar] [CrossRef]
- Shukla, S.; Fu, P.; Gupta, S.J. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Kim, N.D.; Chiu, K.; Franzen, C.A.; Bergan, R.C.; Pelling, J.C. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol. Carcinog. 2008, 47, 686–700. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713. [Google Scholar] [CrossRef]
- Reiner, T.; Parrondo, R.; de las Pozas, A.; Palenzuela, D.; Perez-Stable, C.J. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: Possible role for inhibition of deubiquitinase activity. PLoS ONE 2013, 8, e56234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.; Moradi, F.; Maddalena, L.A.; Ferreira-Tollstadius, B.; Selim, S.; Stuart, J.A. Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells-involvement of prolyl hydroxylase and hypoxia inducible factor-1. Oncol. Lett. 2019, 17, 697–705. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Kumar, P.; Sakthi Priyadarsini, S.; Meenaxshi, C. In vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis linn against human breast carcinoma cell lines. J. Chem. 2013, 1, 150. [Google Scholar]
- Lucena, F.R.; Almeida, E.R.; Aguiar, J.S.; Silva, T.G.; Souza, V.M.; Nascimento, S.C. Cytotoxic, antitumor and leukocyte migration activities of resveratrol and sitosterol present in the hidroalcoholic extract of Cissus sicyoides L., Vitaceae, leaves. Rev. Bras. Farmacog. 2010, 20, 729–733. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-Y.; Wang, C.-Y.; Hwang, T.-L.; Juang, S.-H.; Hung, H.-Y.; Kuo, P.-C.; Chen, P.-J.; Wu, T.-S. The constituents of the stems of Cissus assamica and their bioactivities. Molecules 2018, 23, 2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Said, M.S.; Khalifa, A.S.; Al-Azizi, M.M. Flavonoids from Cissus digitata. Int. J. Pharm. 1991, 29, 281–283. [Google Scholar] [CrossRef]
- Thakur, A.; Jain, V.; Hingorani, L.; Laddha, K. Phytochemical Studies on Cissus quadrangularis Linn. J. Pharm. Res. 2009, 1, 213. [Google Scholar]
- Adesanya, S.A.; Nia, R.; Martin, M.-T.; Boukamcha, N.; Montagnac, A.; Païs, M. Stilbene derivatives from Cissus quadrangularis. J. Nat. Prod. 1999, 62, 1694–1695. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Lombardi, J.A.; Lovato, M.B. Phylogeny of Cissus (Vitaceae) focusing on South American species. Taxon 2014, 63, 287–298. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Chillemi, R.; Tringali, C. Bioassay-Guided Isolation of Antiproliferative Compounds from Grape (Vitis vinifera) Stems. Nat. Prod. Commun. 2008, 4, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.P.; Bonfim-Mendonça, P.d.S.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.S.; Consolaro, M.E. Oxidative stress triggered by Apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid. Med. Cell Longev. 2017, 15, 12745. [Google Scholar] [CrossRef]
- Abate-Shen, C.J. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat. Rev. Cancer 2002, 2, 777. [Google Scholar] [CrossRef]
- Dennis, J.H.; Budhram-Mahadeo, V.; Latchman, D.S. The Brn-3b POU family transcription factor regulates the cellular growth, proliferation, and anchorage dependence of MCF7 human breast cancer cells. Oncogene 2001, 20, 4961. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.H.; Hyde, C.; Reid, I.N.; Hitchcock, I.S.; Hart, C.A.; Gordon Bryden, A.; Villette, J.M.; Stower, M.J.; Maitland, N.J. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 2002, 52, 253–263. [Google Scholar] [CrossRef]
- Jeter, C.R.; Liu, B.; Liu, X.; Chen, X.; Liu, C.; Calhoun-Davis, T.; Repass, J.; Zaehres, H.; Shen, J.; Tang, D.G. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene 2011, 30, 3833–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeter, C.R.; Badeaux, M.; Choy, G.; Chandra, D.; Patrawala, L.; Liu, C.; Calhoun-Davis, T.; Zaehres, H.; Daley, G.Q.; Tang, D.G. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells 2009, 27, 993–1005. [Google Scholar] [CrossRef] [Green Version]
- Runkle, E.A.; Mu, D.J. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.; Ruch, R.J.; Vang, O.J. Resveratrol reverses tumor-promoter-induced inhibition of gap-junctional intercellular communication. Biochem. Biophys. Res. Commun. 2000, 275, 804–809. [Google Scholar] [CrossRef]
- Sareen, D.; Van Ginkel, P.R.; Takach, J.C.; Mohiuddin, A.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3708–3716. [Google Scholar] [CrossRef] [Green Version]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer. 2001, 94, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chung, H.-Y.; Kim, K.-H.; Lee, J.-J.; Kim, K.-W. Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids. J. Cancer Res. Clin. Oncol. 1994, 120, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Chang, C.-C.; Mori, T.; Sato, K.; Ohtsuki, K.; Upham, B.L.; Trosko, J.E. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 2005, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Isoda, H.; Motojima, H.; Onaga, S.; Samet, I.; Villareal, M.O.; Han, J.J. Analysis of the erythroid differentiation effect of flavonoid apigenin on K562 human chronic leukemia cells. Chem. Biol. Interact. 2014, 220, 269–277. [Google Scholar] [CrossRef] [Green Version]



| Peak | RT (min) | Area (%) | Experimental m/z [M−H] − | Molecular Formula | Tentative Compound Identification in METLIN Database |
|---|---|---|---|---|---|
| 1 | 7.685 | 2.55 | 163.0401 | C9H8O3 | Trans-p-coumaric acid |
| 2 | 7.908 | 3.03 | 193.0504 | C10H10O4 | Isoferulic acid |
| 3 | 8.541 | 10.26 | 287.0569 | C15H12O6 | Dihydrokaempferol |
| 4 | 8.567 | 3.44 | 227.0716 | C14H12O3 | Resveratrol |
| 5 | 9.103 | 8.62 | 269.0434 | C15H10O5 | Apigenin |
| 6 | 9.243 | 4.60 | 285.0404 | C15H10O6 | Kaempferol |
| 7 | 9.345 | 10.74 | 299.0561 | C16H12O6 | Chrysoeriol |
| 8 | 9.479 | 3.46 | 271.0612 | C15H12O5 | Naringenin |
| 9 | 11.699 | 22.51 | 471.3475 | C30H48O4 | 2-alpha hydroxyursolic acid |
| 10 | 12.394 | 16.11 | 455.3515 | C30H48O3 | Ursolic acid |
| 11 | 12.471 | 10.12 | 455.3422 | C30H48O3 | Betulinic acid |
| 12 | 14.401 | 3.38 | 253.2161 | C16H28O2 | Hexadecadienoic acid |
| 13 | 14.582 | 1.18 | 279.2348 | C16H32O2 | Octadecadienoic acid |
| Functional Category | Effect of Resveratrol | % | p-Value |
|---|---|---|---|
| Transcription regulation | Upregulation | 19.82 | 2.30 × 10−3 |
| Nucleus | Upregulation | 11.97 | 7.50 × 10−5 |
| Membrane | Downregulation | 9.14 | 1.70 × 10−3 |
| DNA binding | Upregulation | 6.82 | 3.10 × 10−5 |
| Integral component of membrane | Downregulation | 6.56 | 2.00 × 10−2 |
| Plasma membrane | Downregulation | 5.66 | 9.60 × 10−3 |
| Homeobox | Upregulation | 5.53 | 6.60 × 10−4 |
| Cytoplasmic | Downregulation | 5.02 | 3.70 × 10−3 |
| Cell membrane | Downregulation | 4.12 | 3.10 × 10−2 |
| Hydrolase | Upregulation | 3.86 | 3.80 × 10−2 |
| Transport | Downregulation | 3.47 | 1.20 × 10−3 |
| ATP binding | Upregulation | 3.22 | 5.90 × 10−2 |
| Receptor | Downregulation | 2.83 | 5.30 × 10−3 |
| Transducer | Downregulation | 1.93 | 4.30 × 10−3 |
| Endoplasmic reticulum membrane | Downregulation | 1.93 | 5.10 × 10−3 |
| G-protein coupled receptor pathway | Downregulation | 1.93 | 6.50 × 10−3 |
| Cell differentiation | Upregulation | 1.80 | 6.90 × 10−2 |
| Homeodomain−like | Upregulation | 1.54 | 4.50 × 10−3 |
| Mitochondrial inner membrane | Upregulation | 1.54 | 3.50 × 10−2 |
| Ubiquitin protein ligase binding | Upregulation | 1.29 | 1.80 × 10−2 |
| Fractions | Mobile Phase | Pooled Fractions | Weight (g) |
|---|---|---|---|
| 1–28 | Hexane 100% | F1 | 0.1499 |
| 29–36 | Hexane/Ethyl acetate 85:15 | F2 | 1.8797 |
| 37–45 | Hexane/Ethyl acetate 80:20 | F3 | 0.9695 |
| 46–54 | Hexane/Ethyl acetate 70:30 | F4 | 0.4827 |
| 55–72 | Hexane/Ethyl acetate 60:40 | F5 | 2.1402 |
| 73–108 | Hexane/Ethyl acetate 30:70 | F6 | 1.5938 |
| 109–117 | Ethyl acetate 100% | F7 | 0.4561 |
| 118–128 | Ethyl acetate/Methanol 80:20 | F8 | 0.6795 |
| 129–144 | Ethyl acetate/Methanol 70:30 | F9 | 1.3392 |
| 145–168 | Ethyl acetate/Methanol 50:50 | F10 | 4.1135 |
| 169–186 | Ethyl acetate/Methanol 30:70 | F11 | 3.7527 |
| 187–198 | Ethyl acetate/Methanol 20:80 | F12 | 1.5929 |
| 199–210 | Methanol 100% | F13 | 1.2244 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-López, L.F.; Caboni, P.; Arredondo-Espinoza, E.; Carrizales-Castillo, J.J.J.; Balderas-Rentería, I.; Camacho-Corona, M.d.R. Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells. Molecules 2021, 26, 2200. https://doi.org/10.3390/molecules26082200
Méndez-López LF, Caboni P, Arredondo-Espinoza E, Carrizales-Castillo JJJ, Balderas-Rentería I, Camacho-Corona MdR. Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells. Molecules. 2021; 26(8):2200. https://doi.org/10.3390/molecules26082200
Chicago/Turabian StyleMéndez-López, Luis Fernando, Pierluigi Caboni, Eder Arredondo-Espinoza, Juan J. J. Carrizales-Castillo, Isaías Balderas-Rentería, and María del Rayo Camacho-Corona. 2021. "Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells" Molecules 26, no. 8: 2200. https://doi.org/10.3390/molecules26082200
APA StyleMéndez-López, L. F., Caboni, P., Arredondo-Espinoza, E., Carrizales-Castillo, J. J. J., Balderas-Rentería, I., & Camacho-Corona, M. d. R. (2021). Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells. Molecules, 26(8), 2200. https://doi.org/10.3390/molecules26082200