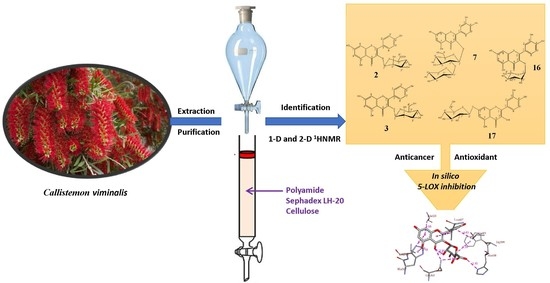
Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structure Elucidation of Compounds Isolated from C. viminalis Aerial Parts
2.1.1. Kaempferol 3-O-(4″-galloyl)-β-d-glucopyranosyl-(1‴→6″)-O-β-d-glucopyranoside (1)
2.1.2. Kaempferol 3-O-β-d-mannuronopyranoside (2) and kaempferol 3-O-β-d-mannopyranoside (3)
2.1.3. Quercetin 3-O-β-d-mannuronopyranoside (4)
2.1.4. 2,3 (S)-hexahydroxydiphenoyl-d-glucose (5)
2.2. Antioxidant Activity
2.2.1. DPPH Radical Scavenging Activity
2.2.2. NO Radical Scavenging Activity
2.2.3. SOD Radical Scavenging Activity
2.3. Cytotoxic Activities of AME and the Pure Isolated Compounds against MCF-7 and HepG2 Cell Lines
2.4. In Silico Analysis for the Antioxidant Effect
3. Materials and Methods
3.1. General Experimental Data
3.2. Plant Material
3.3. Extraction and Isolation
3.4. In Vitro Antioxidant Assays
3.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
3.4.2. Antioxidant Capacity Determined by Nitric Oxide (NO) Scavenging Assay
3.4.3. Antioxidant Capacity Determined by Superoxide Radical (O2−) Scavenging Assay
3.5. In Vitro Cytotoxic Activity
3.6. Data Analysis
3.7. Molecular Modeling Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| HCl | Hydrochloric acid; |
| HepG2 | Hepatoma G2 cell line; |
| IC50 | Concentrations of tested compounds that give about 50% inhibition of cell viability |
| MCF-7 | Michigan Cancer Foundation-7 cell line; |
| MS | Mass Spectrometry; |
| NMR | Nuclear Magnetic Resonance; |
References
- Salem, M.Z.M.; El-Hefny, M.; Nasser, R.A.; Ali, H.M.; El-Shanhorey, N.A.; Elansary, H.O. Medicinal and biological values of Callistemon viminalis extracts: History, current situation and prospects. Asian Pac. J. Trop. Med. 2017, 10, 229–237. [Google Scholar] [CrossRef]
- Ahmad, K.; Athar, F. Phytochemistry and pharmacology of Callistemon viminalis (Myrtaceae): A Review. Nat. Prod. J. 2017, 7, 166–175. [Google Scholar] [CrossRef]
- Salem, M.Z.; Ali, H.M.; El-Shanhorey, N.A.; Abdel-Megeed, A. Evaluation of extracts and essential oil from Callistemon viminalis leaves: Antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac. J. Trop. Med. 2013, 6, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Abd, J. Studying of antibacterial effect for leaves extract of Callistemon viminalis in vitro and vivo (urinary system) for rabbits. J. Kerbala Univ. 2012, 8, 246–254. [Google Scholar]
- Ahmed, A.H. Phytochemical and cytotoxicity studies of Callistemon viminalis leaves extract growing in Egypt. Curr. Pharm. Biotechnol. 2020, 21, 219–225. [Google Scholar] [CrossRef] [PubMed]
- El Dib, R.; El-Shenawy, S. Phenolic constituents and biological activities of the aerial parts of Callistemon viminalis (Sol. Ex Gaertner) G. Don ex Loudon. Bull. Fac. Pharm. 2008, 46, 223–235. [Google Scholar]
- Gohar, A.A.; Maatooq, G.T.; Gadara, S.R.; Aboelmaaty, W.S. One new pyrroline compound from Callistemon viminalis (Sol. Ex Gaertner) G.Don Ex Loudon. Nat. Prod. Res. 2013, 27, 1179–1185. [Google Scholar] [CrossRef]
- Wu, J.W.; Li, B.L.; Tang, C.; Ke, C.Q.; Zhu, N.L.; Qiu, S.X.; Ye, Y. Callistemonols A and B, potent antimicrobial acylphloroglucinol derivatives with unusual carbon skeletons from Callistemon viminalis. J. Nat. Prod. 2019, 82, 1917–1922. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, X.; Liu, R.; Yang, M.; Kong, L.; Luo, J. Acylphloroglucinols from the fruits of Callistemon viminalis. Phytochem. Lett. 2017, 20, 61–65. [Google Scholar] [CrossRef]
- Liu, H.-X.; Chen, K.; Liu, Y.; Li, C.; Wu, J.-W.; Xu, Z.-F.; Tan, H.-B.; Qiu, S.-X. Callviminols AE, new terpenoid-conjugated phloroglucinols from the leaves of Callistemon viminalis. Fitoterapia 2016, 115, 142–147. [Google Scholar] [CrossRef]
- Ahmad, K. 14P Evaluating anti-oxidant potential of Callistemon viminalis leaves extracts and their compounds in STAT 3 pathway in liver cancer. Ann. Oncol. 2017, 28, mdx652.013. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gacche, R.N. Evaluation of anti-breast cancer, anti-angiogenic and antioxidant properties of selected medicinal plants. Eur. J. Integr. Med. 2019, 25, 13–19. [Google Scholar] [CrossRef]
- Hasan, N.; Mamun, A.; Belal, H.; Rahman, A.; Ali, H.; Tasnin, N.; Ara, T.; Rabbi, A.; Asaduzzaman, M.; Islam, A. A report on antioxidant and antibacterial properties of Callistemon viminalis leaf. Int. J. Pharm. Sci. Res. 2016, 1, 36–41. [Google Scholar]
- Tiwari, U.; Jadon, M.; Nigam, D. Evaluation of antioxidant and antibacterial activities of methanolic leaf extract of Calistemon viminalis. Int. J. Pharm. Sci. Bus. Manag. 2014, 2, 1–12. [Google Scholar]
- Bhagat, M.; Sangral, M.; Pandita, S.; Gupta, S.; Bindu, K. Pleiotropic chemodiversity in extracts and Essential oil of Melaleuca viminalis and Melaleuca armillaris of Myrtaceae Family. J. Explor. Res. Pharmacol. 2017, 2, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Abdelhady, M.I.; Youns, M. In-vitro evaluation of the antidiabetic activity of bottle brush plants. RRBS 2014, 9, 134–136. [Google Scholar]
- Shareef, H.; Naeem, S.; Zaheer, E. Comparative analgesic activity of selected medicinal plants from Pakistan. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2019, 56, 57–67. [Google Scholar]
- Mathy-Hartert, M.; Bourgeois, E.; Grulke, S.; Deby-Dupont, G.; Caudron, I.; Deby, C.; Lamy, M.; Serteyn, D. Purification of myeloperoxidase from equine polymorphonuclear leucocytes. Can. J. Vet. Res. 1998, 62, 127–132. [Google Scholar] [PubMed]
- Vellosa, J.C.R.; Regasini, L.O.; Khalil, N.M.; Bolzani, V.d.S.; Khalil, O.A.; Manente, F.A.; Pasquini Netto, H.; Oliveira, O.M. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética Quim. 2011, 36, 7–20. [Google Scholar] [CrossRef]
- Ostuni, M.A.; Gelinotte, M.; Bizouarn, T.; Baciou, L.; Houée-Levin, C. Targeting NADPH-oxidase by reactive oxygen species reveals an initial sensitive step in the assembly process. Free Radic. Biol. Med. 2010, 49, 900–907. [Google Scholar] [CrossRef]
- Cho, K.J.; Seo, J.M.; Kim, J.H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 2011, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Awad, S.M.; Said, A.M.; Mahgoub, S.; Taha, H.; Ahmed, N.M. Design, synthesis, molecular modelling and biological evaluation of novel 3-(2-naphthyl)-1-phenyl-1H-pyrazole derivatives as potent antioxidants and 15-Lipoxygenase inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 847–863. [Google Scholar] [CrossRef]
- Yun, M.R.; Park, H.M.; Seo, K.W.; Lee, S.J.; Im, D.S.; Kim, C.D. 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase. Free Radic. Res. 2010, 44, 742–750. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, M.; Sharma, S.; Bhatti, R.; Singh, P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: Identification of a lead for anti-inflammatory drug. Eur. J. Med. Chem. 2014, 77, 185–192. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Kumar, M.; Shekhar, S.; Rai, N.; Kaur, P.; Parshad, R.; Dey, S. Serum 5-LOX: A progressive protein marker for breast cancer and new approach for therapeutic target. Carcinogenesis 2016, 37, 912–917. [Google Scholar] [CrossRef] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Beebe, J.; Schwartzbaum, J.J.W.A.o.S.J. Chemoprevention of breast cancer by cyclooxygenase and lipoxygenase inhibitors. World Acad. Sci. 2020, 2, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M.; Deng, J.J.; Yuan, G.J.; Yang, F.; Guo, H.T.; Xiang, M.; Ge, W.; Wu, Y.G. 5-Lipoxygenase contributes to the progression of hepatocellular carcinoma. Mol. Med. Rep. 2011, 4, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.X.; Ding, X.L.; Wu, S.B.; Zhang, H.F.; Cao, W.; Qu, L.S.; Zhang, H. Inhibition of 5-lipoxygenase triggers apoptosis in pancreatic cancer cells. Oncol. Rep. 2015, 33, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Steele, V.E.; Holmes, C.A.; Hawk, E.T.; Kopelovich, L.; Lubet, R.A.; Crowell, J.A.; Sigman, C.C.; Kelloff, G.J. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomark. Prev. 1999, 8, 467–483. [Google Scholar]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem. Toxicol. 2008, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Harborne, J.B.; Mabry, T.J. The Flavonoids: Advances in Research; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Mabry, T.J.; Markham, K.; Thomas, M. The Determination and Interpretation of NMR Spectra of Flavonoids. In The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; pp. 253–273. [Google Scholar]
- Webby, R.F. A flavonol triglycoside from Actinidia arguta var. giraldii. Phytochemistry 1991, 30, 2443–2444. [Google Scholar] [CrossRef]
- Iwashina, T.; Kamenosono, K.; Hatta, H. Flavonoid glycosides from leaves of Aucuba japonica and Helwingia japonica (Comaceae): Phytochemical relationship with the genus Cornus. J. Jpn. Bot. 1997, 72, 337–346. [Google Scholar]
- Salman, H.; Ramasamy, S.; Mahmood, B. Detection of caffeic and chlorogenic acids from methanolic extract of Annona squamosa bark by LC-ESI-MS/MS. J. Intercult. Ethnopharmacol. 2018, 7, 76–81. [Google Scholar] [CrossRef] [Green Version]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Barakat, H.H.; Hussein, S.A.; Marzouk, M.S.; Merfort, I.; Linscheid, M.; Nawwar, M.A. Polyphenolic metabolites of Epilobium hirsutum. Phytochemistry 1997, 46, 935–941. [Google Scholar] [CrossRef]
- Nawwar, M.; Ayoub, N.; El-Raey, M.; Zaghloul, S.; Hashem, A.; Mostafa, E.; Eldahshan, O.; Lindequist, U.; Linscheid, M.W. Acylated flavonol diglucosides from Ammania auriculata. Z. Nat. C J. Biosci. 2015, 70, 39–43. [Google Scholar] [CrossRef]
- Gjersing, E.; Happs, R.M.; Sykes, R.W.; Doeppke, C.; Davis, M.F. Rapid determination of sugar content in biomass hydrolysates using nuclear magnetic resonance spectroscopy. Biotechnol. Bioeng. 2013, 110, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Ohyama, Y.; Tomoda, H.; Abe, T.; Namikoshi, M.; Omura, S. Structure elucidation of roselipins, inhibitors of diacylglycerol acyltransferase produced by gliodadium roseum KF-1040. J. Antibiot. 1999, 52, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, P.A.; Mazurek, M. Further studies on the assignment of signals in 13C magnetic resonance spectra of aldoses and derived methyl glycosides. Can. J. Chem. 1975, 53, 1212–1223. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannis of Casuarina and Stachyurus species. Part 1. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. 1 1983, 1765–1772. [Google Scholar] [CrossRef]
- Moharram, F.; Marzouk, M.; El-Toumy, S.; Ahmed, A.; Aboutabl, E. Polyphenols of Melaleuca quinquenervia leaves–pharmacological studies of grandinin. Phytother. Res. 2003, 17, 767–773. [Google Scholar] [CrossRef]
- Vivas, N.; Laguerre, M.; Glories, Y.; Bourgeois, G.; Vitry, C. Structure simulation of two ellagitannins from Quercus robur L. Phytochemistry 1995, 39, 1193–1199. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) Essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Moukette, B.M.; Pieme, C.A.; Njimou, J.R.; Biapa, C.P.N.; Marco, B.; Ngogang, J.Y. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol. Res. 2015, 48, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, D.; Siva, R. Amelioration of oxidative stress in bio-membranes and macromolecules by non-toxic dye from Morinda tinctoria (Roxb.) roots. Food Chem. Toxicol. 2012, 50, 2062–2069. [Google Scholar] [CrossRef]
- Ialenti, A.; Moncada, S.; Di Rosa, M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br. J. Pharm. 1993, 110, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed. Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.J.; Khodr, H.; Hider, C.R.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Alfa, H.H.; Arroo, R.R.J. Over 3 decades of research on dietary flavonoid antioxidants and cancer prevention: What have we achieved? Phytochem. Rev. 2019, 18, 989–1004. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mishra, A.K.; Mishra, A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell. Mol. Biol. 2012, 58, 142–147. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Singh, B.; Arora, S. Amelioration of oxidative damage by methyl gallate in different in vitro models. Phytopharmacology 2011, 1, 82–94. [Google Scholar]
- Hsu, F.L.; Huang, W.J.; Wu, T.H.; Lee, M.H.; Chen, L.C.; Lu, H.J.; Hou, W.C.; Lin, M.H. Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Shabbir, M.; Khan, M.R. Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker. Chem. Cent. J. 2018, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.S.; Moharram, F.A.; Mohamed, M.A.; Gamal-Eldeen, A.M.; Aboutabl, E.A. Anticancer and antioxidant tannins from Pimenta dioica leaves. Z. Nat. C J. Biosci. 2007, 62, 526–536. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Wu, L. Effect of chlorogenic acid on antioxidant activity of Flos lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Examinati, R.R.I.N.; Wulandari, A.P.; Putri Huspa, D.H.; Andayaningsih, P. Cytotoxicity of aromatic compound from an endophytic fungus, Cladosporium sp. En-S01. Int. J. Curr. Pharm. Res. 2018, 10, 10–12. [Google Scholar] [CrossRef] [Green Version]
- McGuire, S. World Cancer Report 2014; World Health Organization, International Agency for Research on Cancer, WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Senawong, T.; Khaopha, S.; Misuna, S.; Komaikul, J.; Senawong, G.; Wongphakham, P.; Yunchalard, S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci. Asia 2014, 40, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Tarannum, S.; Yariswamy, M.; Vivek, H.K.; Siddesha, J.M.; Angaswamy, N.; Vishwanath, B.S. Ascorbic acid 6-palmitate: A potent inhibitor of human and soybean lipoxygenase-dependent lipid peroxidation. J. Pharm. Pharm. 2014, 66, 769–778. [Google Scholar] [CrossRef]
- Borbulevych, O.Y.; Jankun, J.; Selman, S.H.; Skrzypczak-Jankun, E. Lipoxygenase interactions with natural flavonoid, quercetin, reveal a complex with protocatechuic acid in its X-ray structure at 2.1 Å resolution. Proteins Struct. Funct. Bioinform. 2004, 54, 13–19. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiermann, A. Die Untersuchung potentieller Wirkstoffe in Epilobium-Arten. 1. Mitteilung: Aufklärung der Flavonoidmuster. Sci. Pharm. 1983, 51, 158–167. [Google Scholar]
- Gupta, R.K.; Al-Shafi, S.M.K.; Layden, K.; Haslam, E. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 2. Esters of (S)-hexahydroxydiphenic acid with D-glucopyranose (4 C 1). J. Chem. Soc. Perkin Trans. 1 1982, 2525–2534. [Google Scholar] [CrossRef]
- Stahl, E.; Dumont, E. Gradient thin-layer chromatography on defined “Acidic-Basic” slica gel layers. J. Chromatogr. Sci. 1969, 7, 517–525. [Google Scholar] [CrossRef]
- Nahar, L.; Russell, W.R.; Middleton, M.; Shoeb, M.; Sarker, S.D. Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm. 2005, 55, 187–193. [Google Scholar]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafezi, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]





| Carbon No | δ C | δH * | H-MBC |
|---|---|---|---|
| 2 | 156.8 | ||
| 3 | 133.8 | ||
| 4 | 177.8 | ||
| 5 | 161.8 | ||
| 6 | 98.9 | 6.21 (br s) | C-5, C-8, C-10 |
| 7 | 164.6 | ||
| 8 | 94.2 | 6.44 (br s) | C-6, C-7, C-9, C-10 |
| 9 | 156.9 | ||
| 10 | 104.4 | ||
| 1′ | 121.2 | ||
| 2′/6′ | 131.4 | 8.07 (d, 8 Hz) | C-2, C-4′ |
| 4′ | 160.3 | ||
| 3′/5′ | 115.7 | 6.86 (d, 8) | C-1′, C-4′ |
| OH-5 | 12.62 | ||
| 1″ | 102.1 | 5.39 (d, 8) | C-3 |
| 2″ | 74.2 # | 3.52 | |
| 3″ | 74.3 | 3.14 | |
| 4″ | 71.9 | 4.82 | C-3″, C-5″, C-6″, C-7′′′′ |
| 5″ | 71.6 | 3.53 | |
| 6″ | 68.2 | 3.66 | C-1‴ |
| 1‴ | 101.2 | 5.46 (d, 8) | C-6″ |
| 2‴ | 73.5 # | 3.37 | |
| 3‴ | 77.7 | 3.08 | |
| 4‴ | 70.2 | 3.08 | |
| 5‴ | 76.1 | 3.33 | |
| 6‴ | 60.8 | 3.29, 3.44 | |
| 1′′′′ | 119.5 | ||
| 2′′′′/6′′′′ | 109.2 | 6.97 (br s) | C1′′′′, C4′′′′, C3′′′′/5′′′′′, C7′′′′ |
| 3′′′′/5′′′′ | 145.8 | ||
| 4′′′′ | 139.0 | ||
| 7′′′′ | 165.7 |
| Carbon No | 2 | 3 | ||
|---|---|---|---|---|
| δ C | δ H | δ C | δH | |
| 1 | ||||
| 2 | 156.8 | 156.8 | ||
| 3 | 133.5 | 133.9 | ||
| 4 | 177.5 | 177.8 | ||
| 5 | 161.5 | 161.5 | ||
| 6 | 99.4 | 6.16 (brs) | 97.5 | 6.14 (brs) |
| 7 | 164.9 | 164.6 | ||
| 8 | 94.2 | 6.38(brs) | 94.4 | 6.36 (brs) |
| 9 | 156.8 | 156.8 | ||
| 10 | 104.1 | 104.4 | ||
| 1′ | 121.1 | 121.4 | ||
| 2′/6′ | 131.7 | 8.03 (d, 7.2) | 131.7 | 8.03 (d, 6.8) |
| 4′ | 160.6 | 160.3 | ||
| 3′/5′ | 115.6 | 6.86 (d, 7.2) | 115.7 | 6.86 (d, 6.8) |
| OH-5 | 12.48 | 12.49 | ||
| 1″ | 101.6 | 5.45(2.8) | 101.6 | 5.43 (d, 2.4) |
| 2″ | 74.3 | 4.14–3.25 | 72.1 | 3.51–3.24 |
| 3″ | 75.6 | 74.3 | ||
| 4″ | 72.1 | 70.2 | ||
| 5″ | 76.4 | 76.5 | ||
| 6″ | 171.6 | 62.4 | ||
| Carbon No | δ C | δH | HMBC | Carbon No | δ C | δH | HMBC |
|---|---|---|---|---|---|---|---|
| 1 | 66.9 | 5.43(d,4.8) | C-2, C-3′ | 5′ | 137.9 | ||
| 2 | 76.5 | 4.56 (dd, 2, 4) | C-1, C-7′ | 6′ | 145.9 | ||
| 3 | 71.4 | 5.06 (m) | C-7″ | 7′ | 164.3 | ||
| 4 | 71.1 | 3.36(m) | 1″ | 115.7 | |||
| 5 | 74.6 | 3.57(dd, 3.2, 1.6) | C-4 | 2″ | 126.9 | ||
| 6 | 63.2 | 3.34–3.44 (m) | C-5 | 3″ | 103.4 | 6.16(s) | C-2″, C-7″ |
| 1′ | 115.8 | 4″ | 144.3 | ||||
| 2′ | 119.9 | 5″ | 133.9 | ||||
| 3′ | 116.2 | 6″ | 145.3 | ||||
| 4′ | 143.0 | 7″ | 170.6 |
| Compound/Extract | IC50 μg/mL ± SEM (IC50 μM) | ||
|---|---|---|---|
| DPPH | NO | SOD | |
| AME | 25.48 ± 0.29 | 12.67 ± 0.84 | 0.50 ± 0.29 |
| 2 | 6.08 ± 0.05 (13.2) | 10.70 ± 1.58 (23.2) | 10.18 ± 0.49 (22.1) |
| 3 | 8.93 ± 0.17 (19.9) | 11.21 ± 0.47 (25) | 6.05 ± 0.57 (13.6) |
| 4 | 15.86 ± 0.21 (33.3) | 16.55 ± 0.29 (34.7) | 6.00 ± 0.34 (12.6) |
| 7 | 6.51 ± 0.18 (11.4) | 15.10 ± 1.56 (26.5) | 11.58 ± 0.41 (20.4) |
| 10 | 18.13 ± 0.38 (39.2) | 12.03 ± 0.76 (26.0) | 6.76 ± 0.16 (14.7) |
| 15 | 22.14 ± 0.31 (47.6) | 11.51 ± 1.91 (24.8) | 6.06 ± 0.57 (13.1) |
| 16 | 19.39 ± 0.58 (43.3) | 7.84 ± 0.88 (17.4) | 21.48 ± 0.57 (48) |
| 1 7 | 8.94 ± 0.28 (19.2) | 13.13 ± 0.74 (28.2) | 5.27 ± 0.49 (11.4) |
| Ascorbic acid | 19.63 ± 0.37 (111.3) | 12.95 ± 0.77 (73.5) | 21.62 ± 0.40 (122.6) |
| Compound/Extract | IC50 μg/mL ± SEM (IC50 μM) | |
|---|---|---|
| MCF-7 Cell Line | HepG2 Cell Line | |
| AME | 229.0 ± 5.2 | 214.0 ± 5.8 |
| 2 | 224.0 ± 4.6 (484.9) | 175.0 ± 5.4 (378.8) |
| 3 | 85.1 ± 3.1(190.0) | 44.9 ± 2.8 (100.2) |
| 4 | 60.9 ± 1.8 (127.4) | 60.2 ± 2.7 (125.9) |
| 7 | 62.0 ± 1.9 (101.0) | 53.5 ± 3.1 (90.0) |
| 10 | 124.0 ± 2.9 (268.4) | 142.0 ± 5.2 (307.4) |
| 15 | 86.4 ± 2.7 (186.2) | 61.5 ± 2.9 (132.5) |
| 16 | 59.4 ± 2.4 (132.5) | 44.9 ± 2.4 (100.2) |
| 17 | 101.0 ± 3.1 (217.7) | 58.5 ± 2.7 (126.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahgoub, S.; Hashad, N.; Ali, S.; Ibrahim, R.; Said, A.M.; Moharram, F.A.; Mady, M. Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations. Molecules 2021, 26, 2481. https://doi.org/10.3390/molecules26092481
Mahgoub S, Hashad N, Ali S, Ibrahim R, Said AM, Moharram FA, Mady M. Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations. Molecules. 2021; 26(9):2481. https://doi.org/10.3390/molecules26092481
Chicago/Turabian StyleMahgoub, Shahenda, Nashwa Hashad, Sahar Ali, Reham Ibrahim, Ahmed M. Said, Fatma A. Moharram, and Mohamed Mady. 2021. "Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations" Molecules 26, no. 9: 2481. https://doi.org/10.3390/molecules26092481
APA StyleMahgoub, S., Hashad, N., Ali, S., Ibrahim, R., Said, A. M., Moharram, F. A., & Mady, M. (2021). Polyphenolic Profile of Callistemon viminalis Aerial Parts: Antioxidant, Anticancer and In Silico 5-LOX Inhibitory Evaluations. Molecules, 26(9), 2481. https://doi.org/10.3390/molecules26092481