Secretion of Mutant DNA and mRNA by the Exosomes of Breast Cancer Cells
Abstract
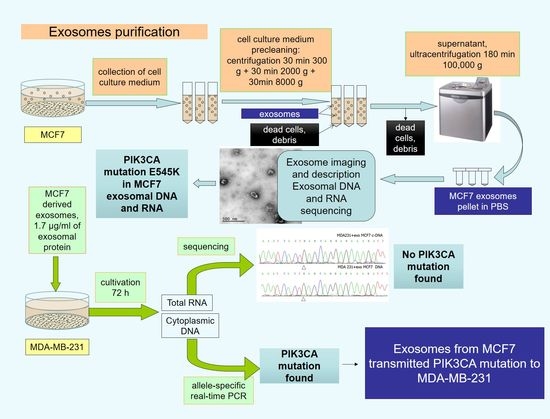
:1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, J.; Morley, S.; Le, M.; Bedoret, D.; Umetsu, D.T.; Di Vizio, D.; Freeman, M.R. Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells: Potential effects on the tumor microenvironment. Cancer Biol. Ther. 2014, 15, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal. Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Tamkovich, S.N.; Yunusova, N.V.; Tugutova, E.; Somov, A.K.; Proskura, K.V.; Kolomiets, L.A.; Stakheeva, M.N.; Grigor’eva, A.E.; Laktionov, P.P.; Kondakova, I.V. Protease Cargo in Circulating Exosomes of Breast Cancer and Ovarian Cancer Patients. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konoshenko, M.; Sagaradze, G.; Orlova, E.; Shtam, T.; Proskura, K.; Kamyshinsky, R.; Yunusova, N.; Alexandrova, A.; Efimenko, A.; Tamkovich, S. Total Blood Exosomes in Breast Cancer: Potential Role in Crucial Steps of Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 7341. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shin, S.; Kim, B.; Lee, K.A. Selecting short length nucleic acids localized in exosomes improves plasma EGFR mutation detection in NSCLC patients. Cancer Cell Int. 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.X.; Li, Y.M.; Ye, M.; Guo, Y.Y.; Li, Q.W.; Peng, X.M.; Wang, Q.; Zhang, S.F.; Zhao, H.X.; Zhang, H.; et al. KRAS and BRAF mutations in serum exosomes from patients with colorectal cancer in a Chinese population. Oncol. Lett. 2017, 13, 3608–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Semina, S.E.; Scherbakov, A.M.; Vnukova, A.A.; Bagrov, D.V.; Evtushenko, E.G.; Safronova, V.M.; Golovina, D.A.; Lyubchenko, L.N.; Gudkova, M.V.; Krasil’nikov, M.A. Exosome-Mediated Transfer of Cancer Cell Resistance to Antiestrogen Drugs. Molecules 2018, 23, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, S.; Cornils, K.; Speiseder, T.; Badbaran, A.; Reimer, R.; Indenbirken, D.; Grundhoff, A.; Brunswig-Spickenheier, B.; Alawi, M.; Lange, C. Indication of Horizontal DNA Gene Transfer by Extracellular Vesicles. PLoS ONE 2016, 11, e0163665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanius, K.; Servage, K.; de Souza Santos, M.; Gray, H.F.; Toombs, J.E.; Chimalapati, S.; Kim, M.S.; Malladi, V.S.; Brekken, R.; Orth, K. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. Elife 2019, 8, e40226. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Han, Y.; Ren, H.; Chen, C.; He, D.; Zhou, L.; Eisner, G.M.; Asico, L.D.; Jose, P.A.; Zeng, C. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J. Mol. Cell Biol. 2013, 5, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Loo, T.M.; Okada, R.; Kamachi, F.; Watanabe, Y.; Wakita, M.; Watanabe, S.; Kawamoto, S.; Miyata, K.; Barber, G.N.; et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 2018, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guan, W.; Tan, X.; Chen, C.; Li, L.; Wang, N.; Zou, X.; Zhou, F.; Wang, J.; Pei, F.; et al. SRY gene transferred by extracellular vesicles accelerates atherosclerosis by promotion of leucocyte adherence to endothelial cells. Clin. Sci. 2015, 129, 259–269. [Google Scholar] [CrossRef] [PubMed]




| Sample | Reference Allele | Mutant Allele | Delta Ct | Delta Ct Cut-off | Mutation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct (1) | Ct (2) | Ct (3) | Average Ct | Ct (1) | Ct (2) | Ct (3) | Average Ct | ||||
| MCF7 cells | 15.1 | 15.3 | 15.7 | 15.4 | 19.1 | 19.2 | 19.2 | 19.2 | 3.8 | 11.5 | Yes |
| MDA-MB-231 cells | 17.4 | 17.4 | 17.4 | 17.4 | 29.7 | 30.2 | 30.5 | 30.1 | 12.7 | No | |
| MDA-MB-231 cells + exoMCF7 | 15.9 | 15.9 | 16 | 15.9 | 26.1 | 26.2 | 26.3 | 26.2 | 10.3 | Yes | |
| RT-PCR negative control | - | - | - | - | - | - | - | - | - | No | |
| Sequence Primers PIK3CA (p.Glu545Lys) | Fragment Length | T | |
|---|---|---|---|
| DNA | F: gggaaaaatatgacaaagaaagc R: ctgagatcagccaaattcagtt | 250 bp | 60 |
| c-DNA | F1: ccacgcaggactgagtaaca R1: ggccaatcttttaccaagca | 246 bp | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, O.E.; Shchegolev, Y.Y.; Scherbakov, A.M.; Mikhaevich, E.I.; Sorokin, D.V.; Gudkova, M.V.; Bure, I.V.; Kuznetsova, E.B.; Mikhaylenko, D.S.; Nemtsova, M.V.; et al. Secretion of Mutant DNA and mRNA by the Exosomes of Breast Cancer Cells. Molecules 2021, 26, 2499. https://doi.org/10.3390/molecules26092499
Andreeva OE, Shchegolev YY, Scherbakov AM, Mikhaevich EI, Sorokin DV, Gudkova MV, Bure IV, Kuznetsova EB, Mikhaylenko DS, Nemtsova MV, et al. Secretion of Mutant DNA and mRNA by the Exosomes of Breast Cancer Cells. Molecules. 2021; 26(9):2499. https://doi.org/10.3390/molecules26092499
Chicago/Turabian StyleAndreeva, Olga E., Yuri Y. Shchegolev, Alexander M. Scherbakov, Ekaterina I. Mikhaevich, Danila V. Sorokin, Margarita V. Gudkova, Irina V. Bure, Ekaterina B. Kuznetsova, Dmitry S. Mikhaylenko, Marina V. Nemtsova, and et al. 2021. "Secretion of Mutant DNA and mRNA by the Exosomes of Breast Cancer Cells" Molecules 26, no. 9: 2499. https://doi.org/10.3390/molecules26092499
APA StyleAndreeva, O. E., Shchegolev, Y. Y., Scherbakov, A. M., Mikhaevich, E. I., Sorokin, D. V., Gudkova, M. V., Bure, I. V., Kuznetsova, E. B., Mikhaylenko, D. S., Nemtsova, M. V., Bagrov, D. V., & Krasil’nikov, M. A. (2021). Secretion of Mutant DNA and mRNA by the Exosomes of Breast Cancer Cells. Molecules, 26(9), 2499. https://doi.org/10.3390/molecules26092499