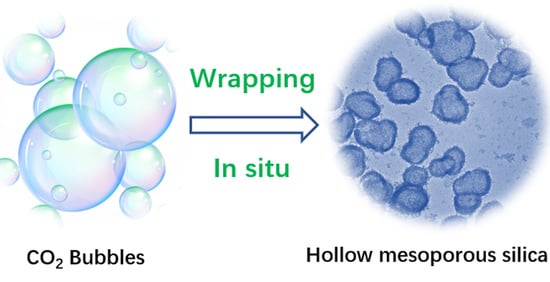
Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Amino-Functionalized Mesoporous Silica Hollow Spheres
3.2. Carbon Dioxide Capture Experiment
3.3. Knoevenagel Reaction
3.4. Metoprolol Tartrate (MPT) Drug-loading and Release Experiments
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ryoo, R. Birth of a class of nanomaterial. Nature 2019, 575, 40–41. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Williams, G.R.; Niu, S.; Gao, F.; Tang, R.; Zhu, L.M. A multifunctional biodegradable nanocomposite for cancer theranostics. Adv. Sci. 2019, 6, 1802001. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Cosco, E.D.; Ramakrishnan, S.; Lingg, J.G.P.; Bruns, O.T.; Zink, J.I.; Sletten, E.M. Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc. 2019, 141, 12475–12480. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Wei, Y.; El-Toni, A.M.; Zhang, F.; Zhao, D. Anisotropic encapsulation-induced synthesis of asymmetric single-hole mesoporous nanocages. J. Am. Chem. Soc. 2015, 137, 5903–5906. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Li, F.; Zhou, H.-J.; Sun, P.-C.; Ding, D.-T.; Chen, T.-H. Silica hollow spheres with ordered and radially oriented amino-functionalized mesochannels. Chem. Mater. 2009, 21, 612–620. [Google Scholar] [CrossRef]
- Djojoputro, H.; Zhou, X.F.; Qiao, S.Z.; Wang, L.Z.; Yu, C.Z.; Lu, G.Q. Periodic mesoporous organosilica hollow spheres with tunable wall thickness. J. Am. Chem. Soc. 2006, 128, 6320–6321. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Zhou, X.; Yuan, P.; Yu, C. One template synthesis of raspberry-like hierarchical siliceous hollow spheres. J. Am. Chem. Soc. 2007, 129, 14576–14577. [Google Scholar] [CrossRef]
- Yeh, Y.-Q.; Chen, B.-C.; Lin, H.-P.; Tang, C.-Y. Synthesis of hollow silica spheres with mesostructured shell using cationic− anionic-neutral block copolymer ternary surfactants. Langmuir 2006, 22, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.K.; Mastai, Y.; Gedanken, A. Acoustic cavitation leading to the morphosynthesis of mesoporous silica vesicles. Adv. Mater. 2002, 14, 1414–1418. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, M.; Yu, K.; Zhang, S.; Xie, Y. Self-Assembled Double-Shelled Ferrihydrite Hollow Spheres with a Tunable Aperture. Chem.-Eur. J. 2008, 14, 5346–5352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Li, W.; Zhang, C.; Han, B. Synthesis of uniform hollow silica spheres with ordered mesoporous shells in a CO2 induced nanoemulsion. Chem. Commun. 2009, 2365–2367. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Wang, D.; Guo, R.; Li, X.; Qi, W. Interfacially controlled synthesis of hollow mesoporous silica spheres with radially oriented pore structures. Langmuir 2010, 26, 12267–12272. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Wu, S.-H.; Tseng, C.-T.; Hung, Y.; Chang, C.; Mou, C.-Y. Synthesis of hollow silica nanospheres with a microemulsion as the template. Chem. Commun. 2009, 3542–3544. [Google Scholar] [CrossRef]
- Fujiwara, M.; Shiokawa, K.; Sakakura, I.; Nakahara, Y. Silica hollow spheres with nano-macroholes like diatomaceous earth. Nano Lett. 2006, 6, 2925–2928. [Google Scholar] [CrossRef] [PubMed]
- Meka, A.K.; Abbaraju, P.L.; Song, H.; Xu, C.; Zhang, J.; Zhang, H.; Yu, M.; Yu, C. A vesicle supra-assembly approach to synthesize amine-functionalized hollow dendritic mesoporous silica nanospheres for protein delivery. Small 2016, 12, 5169–5177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, M.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. One-step production of amine-functionalized hollow mesoporous silica microspheres via phase separation-induced cavity in miniemulsion system for opaque and matting coating. Ind. Eng. Chem. Res. 2020, 59, 723–731. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Li, T.; Zheng, H.; Xu, Y.; Chen, J.; Wang, J. Multipodal mesoporous silica hollow spheres: Branched hierarchical nanostructure by region-selective self-assembly. J. Colloid Interf. Sci. 2020, 579, 21–27. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, S.-H. Polyelectrolyte controlled large-scale synthesis of hollow silica spheres with tunable sizes and wall thicknesses. J. Phys. Chem. C 2008, 112, 3641–3647. [Google Scholar] [CrossRef]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials. ACS Appl. Mater. Inter. 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoi, T.; Hideaki, Y.; Takashi, T. Synthesis of Amino-Functionalized MCM-41 Via Direct Co-Condensation and Post-Synthesis Grafting Methods Using Mono-, Di- and Tri-Amino-Organoalkoxysilanes. J. Mater. Chem. 2004, 14, 951–957. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, J.; Shen, W.H.; Chen, H.; Dong, X.; Ruan, M.L. Preparation of novel hollow mesoporous silica spheres and their sustained-release property. Nanotechnology 2005, 16, 2633–2638. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, X.; Saliy, O.; Hu, W.; Wang, J. Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles. Molecules 2022, 27, 53. https://doi.org/10.3390/molecules27010053
Wang H, Liu X, Saliy O, Hu W, Wang J. Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles. Molecules. 2022; 27(1):53. https://doi.org/10.3390/molecules27010053
Chicago/Turabian StyleWang, Hongjuan, Xuefei Liu, Olena Saliy, Wei Hu, and Jingui Wang. 2022. "Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles" Molecules 27, no. 1: 53. https://doi.org/10.3390/molecules27010053
APA StyleWang, H., Liu, X., Saliy, O., Hu, W., & Wang, J. (2022). Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles. Molecules, 27(1), 53. https://doi.org/10.3390/molecules27010053