Porphyrin NanoMetal-Organic Frameworks as Cancer Theranostic Agents
Abstract
:1. Introduction
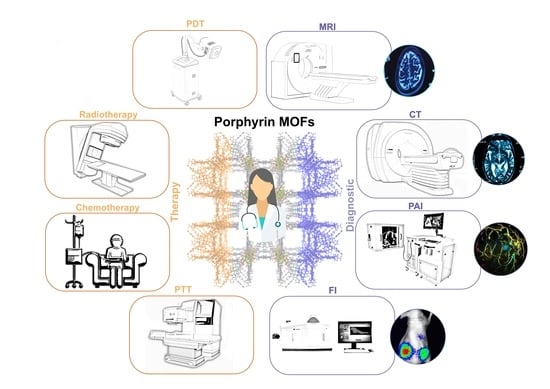
2. Nano-sized Porphyrin MOFs Cancer Theranostic Modes
2.1. Cancer Therapy Modes
2.2. Cancer Imaging Techniques
3. Porphyrin MOFs as Theranostic Agents
3.1. Porphyrin Inclusion into NMOFs
3.2. Porphyrin NMOFs
3.3. Porphyrin NMOF Sheets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNR | Gold nanorods |
| BTC | Benzene 1,3,5-tricarboxylate |
| Cy3 | Cyanine 3 |
| DOX | Doxorubicin |
| CPT | Camptothecin |
| CT | Computed Tomography |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FA | Folate |
| FI | Fluorescence imaging |
| GSH | Glutathione |
| H2TMPyP | tetrakis(1-methylpyridinium-4-yl)porphyrin |
| H2TPyP | tetrakis(4-pyridyl)porphyrin |
| H2TAPC | tetrakis(4-aminophenyl)chlorin |
| H6TCPC | tetrakis(4-carboxyphenyl)chlorin |
| H6TCPP | tetrakis(4-carboxyphenyl)porphyrin |
| HKUST | Hong Kong University of Science and Technology |
| LED | Light-emitting diode |
| MRI | Magnetic Resonance Imaging |
| MOF | Metal-Organic Frameworks |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NIR | Near-Infrared |
| NMOF | nano Metal-Organic Frameworks |
| NHS | N-hydroxysuccinimide |
| PdPTP | Palladium-tetrakis-(4-carbonylphenyl)-tetrabenzoporphyrin |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| PAI | Photoacoustic imaging |
| PCN | Porous Coordination Polymer |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SPECT | Single-photon emission computer tomography |
| SNO | S-nitrosothiol |
| SPION | Superparamagnetic iron oxide nanoparticles |
| TAMRA | 5-carboxytetramethylrhodamine |
| UiO | Universitetet i Oslo |
References
- Pandey, A.P.; Girase, N.M.; Patil, M.D.; Patil, P.O.; Patil, D.A.; Deshmukh, P.K. Nanoarchitectonics in cancer therapy and imaging diagnosis. J. Nanosci. Nanotechnol. 2014, 14, 828–840. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Delivery Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Minelli, C.; Lowe, S.B.; Stevens, M.M. Engineering nanocomposite materials for cancer therapy. Small 2010, 6, 2336–2357. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, J.S.; Mendes, R.F.; Figueira, F.; Gaspar, V.M.; Mano, J.F.; Braga, S.S.; Rocha, J.; Almeida Paz, F.A. Bone Tissue Disorders: Healing Through Coordination Chemistry. Chem. Eur. J. 2020, 26, 15416–15437. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.y.; Zare, E.N.; Borzacchiello, A.; Niu, L.n.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Liu, W.L.; Zou, M.Z.; Qin, S.Y.; Cheng, Y.J.; Ma, Y.H.; Sun, Y.X.; Zhang, X.Z. Recent advances of cell membrane-coated nanomaterials for biomedical applications. Adv. Funct. Mater. 2020, 30, 2003559. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdelrahman, I.Y.; El-Bastawisy, H.S.; Mohamed, A.E.; Mosallam, F.M.; Nasser, H.A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; et al. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B 2019, 180, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Figueira, F.; Barbosa, J.S.; Mendes, R.F.; Braga, S.S.; Almeida Paz, F.A. Virus meet metal-organic frameworks: A nanoporous solution to a world-sized problem? Mater. Today 2021, 43, 84–98. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Soleymaniha, M.; Shahbazi, M.A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting role of MXene nanosheets in biomedical sciences: Therapeutic and biosensing innovations. Adv. Funct. Mater. 2019, 8, 1801137. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO 2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliev, T. The Advances in Biomedical Applications of Carbon Nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, S.; Xia, X.; Zhu, Z.; Chen, L.; Chen, Z. Recent advances in multifunctional graphitic nanocapsules for Raman detection, imaging, and therapy. Small Methods 2020, 4, 1900440. [Google Scholar] [CrossRef]
- Bhunia, S.; Deo, K.A.; Gaharwar, A.K. 2D covalent organic frameworks for biomedical applications. Adv. Funct. Mater. 2020, 30, 2002046. [Google Scholar] [CrossRef]
- Jung, Y.; Huh, Y.; Kim, D. Recent advances in surface engineering of porous silicon nanomaterials for biomedical applications. Microporous Mesoporous Mater. 2021, 310, 110673. [Google Scholar] [CrossRef]
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2020, 31, 1060–1070. [Google Scholar] [CrossRef]
- Leite, J.P.; Rodrigues, D.; Ferreira, S.; Figueira, F.; Almeida Paz, F.A.; Gales, L. Mesoporous Metal–Organic Frameworks as Effective Nucleating Agents in Protein Crystallography. Cryst. Growth Des. 2019, 19, 1610–1615. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Beyond pristine metal-organic frameworks: Preparation and application of nanostructured, nanosized, and analogous MOFs. Coord. Chem. Rev. 2018, 376, 20–45. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Z.; Xu, G. Metal-organic framework nanosheets: Preparation and applications. Coord. Chem. Rev. 2019, 388, 79–106. [Google Scholar] [CrossRef]
- Bieniek, A.; Terzyk, A.P.; Wiśniewski, M.; Roszek, K.; Kowalczyk, P.; Sarkisov, L.; Keskin, S.; Kaneko, K. MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: Recent advances and perspectives. Prog. Mater Sci. 2021, 117, 100743. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Paz, F.A.A. Metal–organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef]
- Zhong, X.-f.; Sun, X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacologica Sinica 2020, 41, 928–935. [Google Scholar] [CrossRef]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef] [Green Version]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Verpoort, F.; Voskressensky, L.G.; Luque, R. Metal-Organic Frameworks (MOFs) for Cancer Therapy. Materials 2021, 14, 7277. [Google Scholar] [CrossRef]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; et al. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef]
- Rajasree, S.S.; Li, X.; Deria, P. Physical properties of porphyrin-based crystalline metal—organic frameworks. Commun. Chem. 2021, 4, 47. [Google Scholar] [CrossRef]
- Figueira, F.; Paz, F.A.A. Porphyrin MOF-Derived Porous Carbons: Preparation and Applications. C 2021, 7, 47. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Shahat, M.; Abd El-Ghaffar, M.A. Boosting the photocatalytic activity of Ti-MOF via emerging with metal phthalocyanine to degrade hazard textile pigments. J. Alloys Compd. 2022, 896, 162992. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [Green Version]
- Figueira, F.; Lourenço, L.M.O.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Tomé, J.P.C. Synthesis and characterization of novel 5-monocarbohydrate-10,20-bis-aryl-porphyrins. J. Porphyrins Phthalocyanines 2019, 24, 330–339. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Figueira, F.; Almeida Paz, F.A.; Tomé, J.P.C.; da Silva, R.S.; Nakagaki, S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Simões, M.M.Q. Copper-phthalocyanine coordination polymer as a reusable catechol oxidase biomimetic catalyst. Dalton Trans. 2019, 48, 8144–8152. [Google Scholar] [CrossRef]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-based compounds and their applications in materials and medicine. Dyes Pigm. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Figueira, F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Silica nanoparticles functionalized with porphyrins and analogs for biomedical studies. J. Porphyrins Phthalocyanines 2011, 15, 517–533. [Google Scholar] [CrossRef]
- Figueira, F.; Rodrigues, J.M.M.; Farinha, A.A.S.; Cavaleiro, J.A.S.; Tomé, J.P.C. Synthesis and anion binding properties of porphyrins and related compounds. J. Porphyrins Phthalocyanines 2016, 20, 950–965. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 3358. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications. Drug Discov. 2017, 22, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, X.; Dai, Z. Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Figueira, F.; Mendes, R.F.; Almeida Paz, F.A.; Neves, M.d.G.P.M.S.; Cavaleiro, J.A.S.; Nakagaki, S.; Tomé, J.P.C.; Simões, M.M.Q. Porphyrinic coordination polymer-type materials as heterogeneous catalysts in catechol oxidation. Polyhedron 2019, 158, 478–484. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal-organic framework-based cancer theranostic nanoplatforms. View 2020, 1, e20. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic porphyrin framework compounds. Trends in Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Ladomenou, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical hydrogen generation with porphyrin-based systems. Coord. Chem. Rev. 2015, 304, 38–54. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Pereira, C.F.; Simões, M.M.; Tomé, J.P.; Almeida Paz, F.A. Porphyrin-based metal-organic frameworks as heterogeneous catalysts in oxidation reactions. Molecules 2016, 21, 1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent advances in porphyrin-based materials for metal ions detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Shan, X.; Zhang, X.; Wang, C.; Zhao, Z.; Zhang, S.; Wang, Y.; Sun, B.; Luo, C.; He, Z. Molecularly engineered carrier-free co-delivery nanoassembly for self-sensitized photothermal cancer therapy. J. Nanobiotechnol. 2021, 19, 282. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Li, X.-Y.; Tan, L.-C.; Dong, L.-W.; Zhang, W.-Q.; Shen, X.-X.; Lu, X.; Zheng, H.; Lu, Y.-G. Susceptibility and Resistance Mechanisms during Photodynamic Therapy of Melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Zhen, W.; Zhang, Q.; Li, Y.; Luo, H.; He, J.; Liu, Y. Porphyrin-Based Metal-Organic Framework Compounds as Promising Nanomedicines in Photodynamic Therapy. ChemMedChem 2020, 15, 1766–1775. [Google Scholar] [CrossRef]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Xu, L.; Zhang, Y.; Timashev, P.S.; Huang, Y.; Liang, X.-J. Polymer-Based Nanomaterials for Noninvasive Cancer Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4289–4305. [Google Scholar] [CrossRef]
- Hak, A.; Ravasaheb Shinde, V.; Rengan, A.K. A review of advanced nanoformulations in phototherapy for cancer therapeutics. Photodiagn. Photodyn. Ther. 2021, 33, 102205. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic photothermal nanoparticles for biomedical applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Liu, B.; Kuang, Y.; Gulzar, A.; He, F.; Gai, S.; Yang, P.; Lin, J. Recent advances in porphyrin-based MOFs for cancer therapy and diagnosis therapy. Coord. Chem. Rev. 2021, 439, 213945. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal–Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef] [Green Version]
- Ni, K.; Lan, G.; Chan, C.; Quigley, B.; Lu, K.; Aung, T.; Guo, N.; La Riviere, P.; Weichselbaum, R.R.; Lin, W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9, 2351. [Google Scholar] [CrossRef]
- Lee, G.; Harnett, N.; Zychla, L.; Dinniwell, R.E. Radiotherapy Treatment Review: A Prospective Evaluation of Concordance between Clinical Specialist Radiation Therapist and Radiation Oncologist in Patient Assessments. J. Med. Imaging Radiat. Sci. 2012, 43, 6–10. [Google Scholar] [CrossRef]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lindstrom, A.; Li, Y. Porphyrin-based nanomedicines for cancer treatment. Bioconjugate Chem. 2019, 30, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ren, X.; Meng, X.; Li, H. Metal-Organic Frameworks-Based Fluorescent Nanocomposites for Bioimaging in Living Cells and in vivo. Chin. J. Chem. 2021, 39, 473–487. [Google Scholar] [CrossRef]
- Grover, V.P.B.; Tognarelli, J.M.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J Clin Exp Hepatol 2015, 5, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Vijayalaxmi; Fatahi, M.; Speck, O. Magnetic resonance imaging (MRI): A review of genetic damage investigations. Mutat. Res. Rev. Mutat. Res. 2015, 764, 51–63. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C.; Castro, M.M.C.A.; Peters, J.A. Mn(III) porphyrins as potential MRI contrast agents for diagnosis and MRI-guided therapy. Coord. Chem. Rev. 2021, 445, 214069. [Google Scholar] [CrossRef]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Calvete, M.J.F.; Pinto, S.M.A.; Pereira, M.M.; Geraldes, C.F.G.C. Metal coordinated pyrrole-based macrocycles as contrast agents for magnetic resonance imaging technologies: Synthesis and applications. Coord. Chem. Rev. 2017, 333, 82–107. [Google Scholar] [CrossRef]
- Seeram, E. Computed Tomography: A Technical Review. Radiol. Technol. 2018, 89, 279ct–302ct. [Google Scholar]
- Lusic, H.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [Green Version]
- Fathi, P.; Pan, D. Current trends in pyrrole and porphyrin-derived nanoscale materials for biomedical applications. Nanomedicine 2020, 15, 2493–2515. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Wojtas, L.; Eddaoudi, M.; Zaworotko, M.J. Template-Directed Synthesis of Nets Based upon Octahemioctahedral Cages That Encapsulate Catalytically Active Metalloporphyrins. J. Am. Chem. Soc. 2012, 134, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, J.; Ma, F.; Ling, P.; Liu, J.; Ju, H. A porphyrin photosensitized metal–organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem. Commun. 2015, 51, 10831–10834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, L.; Liu, M.; Lei, P.; Liu, F.; Xie, Z. Nanoscale Mixed-Component Metal–Organic Frameworks with Photosensitizer Spatial-Arrangement-Dependent Photochemistry for Multimodal-Imaging-Guided Photothermal Therapy. Chem. Mater. 2018, 30, 6867–6876. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, L.; Guan, Y.; Pei, Q.; Jiang, J.; Xie, Z. Integration of metal-organic framework with a photoactive porous-organic polymer for interface enhanced phototherapy. Biomaterials 2020, 235, 119792. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.-M.; Li, Y.-H.; Cai, S.-J.; Yin, X.-B.; He, X.-W.; Zhang, Y.-K. Fluorescent Imaging-Guided Chemotherapy-and-Photodynamic Dual Therapy with Nanoscale Porphyrin Metal–Organic Framework. Small 2017, 13, 1603459. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Xia, L.; Cui, C.; Wan, S.; Jiang, Y.; Yang, Y.; Wu, Q.; Qiu, L.; Tan, W. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem. Sci. 2018, 9, 7505–7509. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, W.; Shang, Y.; Cao, P.; Cui, J.; Li, Z.; Yin, X.; Li, Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: Fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int. J. Nanomed. 2019, 14, 57. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Chen, Y.; Tao, C.; Tian, Q.; An, L.; Lin, J.; Tian, Q.; Yang, H.; Yang, S. Mn–Porphyrin-Based Metal–Organic Framework with High Longitudinal Relaxivity for Magnetic Resonance Imaging Guidance and Oxygen Self-Supplementing Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41946–41956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, X.-T.; Shang, Y.; Li, Y.-H.; Yin, X.-B. Theranostic Mn-Porphyrin Metal–Organic Frameworks for Magnetic Resonance Imaging-Guided Nitric Oxide and Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm Nanotechnol 2019, 7, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Y.S.; Jin, Z.; Zhao, P.; Zhang, H.; Wen, Y.; He, Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horiz. 2019, 4, 1185–1193. [Google Scholar] [CrossRef]
- Bao, J.; Zu, X.; Wang, X.; Li, J.; Fan, D.; Shi, Y.; Xia, Q.; Cheng, J. Multifunctional Hf/Mn-TCPP metal-organic framework nanoparticles for triple-modality imaging-guided PTT/RT synergistic cancer therapy. Int. J. Nanomed. 2020, 15, 7687. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, C.; Li, Z.; Hu, L.; Wei, J.; Tian, J. Defect-engineered porphyrinic metal–organic framework nanoparticles for targeted multimodal cancer phototheranostics. Chem. Commun. 2021, 57, 4035–4038. [Google Scholar] [CrossRef]
- Xie, B.-R.; Yu, Y.; Liu, X.-H.; Zeng, J.-Y.; Zou, M.-Z.; Li, C.-X.; Zeng, X.; Zhang, X.-Z. A near infrared ratiometric platform based π-extended porphyrin metal-organic framework for O2 imaging and cancer therapy. Biomaterials 2021, 272, 120782. [Google Scholar] [CrossRef]
- Yao, S.; Wang, Z.; Li, L. Application of organic frame materials in cancer therapy through regulation of tumor microenvironment. Smart Mater. Med. 2022, 3, 230–242. [Google Scholar] [CrossRef]
- Wan, S.-S.; Cheng, Q.; Zeng, X.; Zhang, X.-Z. A Mn(III)-Sealed Metal–Organic Framework Nanosystem for Redox-Unlocked Tumor Theranostics. ACS Nano 2019, 13, 6561–6571. [Google Scholar] [CrossRef]
- Tian, X.-T.; Cao, P.-P.; Zhang, H.; Li, Y.-H.; Yin, X.-B. GSH-activated MRI-guided enhanced photodynamic- and chemo-combination therapy with a MnO2-coated porphyrin metal organic framework. Chem. Commun. 2019, 55, 6241–6244. [Google Scholar] [CrossRef]
- Zeng, J.-Y.; Zhang, M.-K.; Peng, M.-Y.; Gong, D.; Zhang, X.-Z. Porphyrinic Metal–Organic Frameworks Coated Gold Nanorods as a Versatile Nanoplatform for Combined Photodynamic/Photothermal/Chemotherapy of Tumor. Adv. Funct. Mater. 2018, 28, 1705451. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.-H.; Chen, Y.; Wang, M.-M.; Wang, X.-S.; Yin, X.-B. Fluorescence and Magnetic Resonance Dual-Modality Imaging-Guided Photothermal and Photodynamic Dual-Therapy with Magnetic Porphyrin-Metal Organic Framework Nanocomposites. Sci. Rep. 2017, 7, 44153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakshinamoorthy, A.; Navalón, S.; Asiri, A.M.; Garcia, H. Gold-Nanoparticle-Decorated Metal-Organic Frameworks for Anticancer Therapy. ChemMedChem 2020, 15, 2236–2256. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ai, F.; Han, L. Recent Development of MOF-Based Photothermal Agent for Tumor Ablation. Front. Chem. 2022, 10, 841316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.; Liu, P.; Wang, J.; Yi, C.; Xu, Z.; Ren, J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1–T2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Ma, Q.; Huang, Y.; Zhang, X.; Ping, J.; Zhang, Z.; Lu, Q.; Yu, Y.; Xu, H. Ultrathin 2D metal–organic framework nanosheets. Adv. Mater. 2015, 27, 7372–7378. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Pei, R. Micron-Sized Ultrathin Metal–Organic Framework Sheet. J. Am. Chem. Soc. 2020, 142, 10331–10336. [Google Scholar] [CrossRef]
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Liu, Y.; Liu, D.; Chen, C.; Lu, C.; Zhuang, S.; Kumar, A.; Liu, J. Recent advances in Cu(II)/Cu(I)-MOFs based nano-platforms for developing new nano-medicines. J. Inorg. Biochem. 2021, 225, 111599. [Google Scholar] [CrossRef]
- Xu, M.; Yang, S.S.; Gu, Z.Y. Two-dimensional metal-organic framework nanosheets: A rapidly growing class of versatile nanomaterials for gas separation, MALDI-TOF matrix and biomimetic applications. Chem. Eur. J. 2018, 24, 15131–15142. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, Y.; Jin, Q.; Chao, Y.; Tian, L.; Liu, J.; Dong, Z.; Liu, Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemo-photodynamic cancer therapy. Nano Res. 2019, 12, 1307–1312. [Google Scholar] [CrossRef]
- Morais, M.; Ferreira, V.F.C.; Figueira, F.; Mendes, F.; Raposinho, P.; Santos, I.; Oliveira, B.L.; Correia, J.D.G. Technetium-99m complexes of l-arginine derivatives for targeting amino acid transporters. Dalton Trans. 2017, 46, 14537–14547. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, F.; Tomé, J.P.C.; Paz, F.A.A. Porphyrin NanoMetal-Organic Frameworks as Cancer Theranostic Agents. Molecules 2022, 27, 3111. https://doi.org/10.3390/molecules27103111
Figueira F, Tomé JPC, Paz FAA. Porphyrin NanoMetal-Organic Frameworks as Cancer Theranostic Agents. Molecules. 2022; 27(10):3111. https://doi.org/10.3390/molecules27103111
Chicago/Turabian StyleFigueira, Flávio, João P. C. Tomé, and Filipe A. Almeida Paz. 2022. "Porphyrin NanoMetal-Organic Frameworks as Cancer Theranostic Agents" Molecules 27, no. 10: 3111. https://doi.org/10.3390/molecules27103111
APA StyleFigueira, F., Tomé, J. P. C., & Paz, F. A. A. (2022). Porphyrin NanoMetal-Organic Frameworks as Cancer Theranostic Agents. Molecules, 27(10), 3111. https://doi.org/10.3390/molecules27103111