Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and In Vitro Studies in THP-1 Cells
Abstract
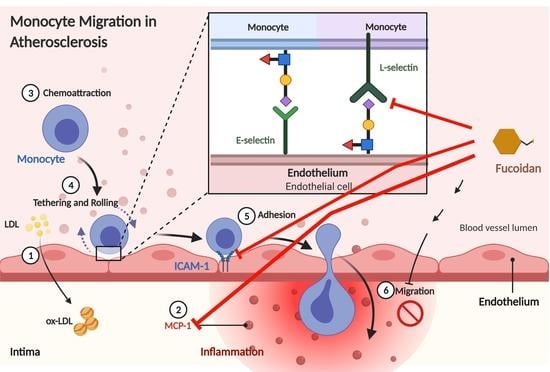
:1. Introduction
2. Results
2.1. Protein–Protein Interaction
2.2. Chemoinformatic Analysis
2.3. Molecular Docking and Potential Binding Site Prediction
2.4. Effect of Fucoidan on Viability and Proliferation of THP-1 Macrophages
2.5. Fucoidan Inhibits Monocytes Migration to MCP-1
2.6. Fucoidan Modulates the Expression of Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Protein–Protein Interaction Study
4.2. Chemoinformatic Prediction
4.3. Molecular Docking
4.4. Cell Culture
4.4.1. Cell Viability and Proliferation Assays
4.4.2. Migration Assay
4.4.3. Quantitative Reverse Transcription-PCR
4.4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Flynn, M.C.; Pernes, G.; Lee, M.K.S.; Nagareddy, P.R.; Murphy, A.J. Monocytes, macrophages, and metabolic disease in atherosclerosis. Front. Pharmacol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Lasky, L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 1995, 64, 113–140. [Google Scholar] [CrossRef]
- Wedepohl, S.; Dernedde, J.; Vahedi-Faridi, A.; Tauber, R.; Saenger, W.; Bulut, H. Reducing Macro- and Microheterogeneity of N-Glycans Enables the Crystal Structure of the Lectin and EGF-Like Domains of Human L-Selectin To Be Solved at 1.9 Å Resolution. ChemBioChem 2017, 18, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Adrielle Lima Vieira, R.; Nascimento de Freitas, R.; Volp, A.C.P. Adhesion molecules and chemokines; relation to anthropometric, body composition, biochemical and dietary variables. Nutr. Hosp. 2014, 30, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Szabó-Fodor, J.; Bónai, A.; Bóta, B.; Szommerné Egyed, L.; Lakatos, F.; Pápai, G.; Zsolnai, A.; Glávits, R.; Horvatovich, K.; Kovács, M. Physiological Effects of Whey- and Milk-Based Probiotic Yogurt in Rats. Polish J. Microbiol. 2017, 66, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Takahashi, K.; Abe, S.; Yamada, K.; Suzuki, M.; Masahisa, M.; Endo, M.; Abe, K.; Inoue, R.; Hoshi, H. Improvement of Psoriasis by Alteration of the Gut Environment by Oral Administration of Fucoidan from Cladosiphon Okamuranus. Mar. Drugs 2020, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of Alginate Extracted from Moroccan Brown Algae to Stimulate Natural Defense in Date Palm Roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wu, S.-Y.; Chen, L.; Li, Q.-J.; Shen, Y.-Z.; Jin, L.; Zhang, X.; Chen, P.-C.; Wu, M.-J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-S.; Kim, E. Fucoidan from Seaweed Fucus vesiculosus Inhibits Migration and Invasion of Human Lung Cancer Cell via PI3K-Akt-mTOR Pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Moumbock, A.F.A.; Li, J.; Mishra, P.; Gao, M.; Günther, S. Current computational methods for predicting protein interactions of natural products. Comput. Struct. Biotechnol. J. 2019, 17, 1367–1376. [Google Scholar] [CrossRef]
- Chen, L.-M.; Tseng, H.-Y.; Chen, Y.-A.; Tanzih, A.; Haq, A.; Hwang, P.-A.; Hsu, H.-L. Oligo-Fucoidan Prevents M2 Macrophage Differentiation and HCT116 Tumor Progression. Cancers 2020, 12, 421. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Yeom, M.; Hahm, D.H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-κB pathway in M2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.I.; Chambers, J.D.; Butcher, E.; Sklar, L.A. Neutrophil aggregation is beta 2-integrin- and L-selectin-dependent in blood and isolated cells. J. Immunol. 1992, 149, 2765–2771. [Google Scholar] [PubMed]
- Bargatze, R.F.; Kurk, S.; Butcher, E.C.; Jutila, M.A. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J. Exp. Med. 1994, 180, 1785–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansas, G.S. Selectins and their ligands: Current concepts and controversies. Blood 1996, 88, 3259–3287. [Google Scholar] [CrossRef] [Green Version]
- Pouyani, T.; Seed, B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell 1995, 83, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Sako, D.; Comess, K.M.; Barone, K.M.; Camphausen, R.T.; Cumming, D.A.; Shaw, G.D. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell 1995, 83, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Peired, A.J.; Wild, M.K.; Vestweber, D.; Frenette, P.S. Complete Identification of E-Selectin Ligands on Neutrophils Reveals Distinct Functions of PSGL-1, ESL-1, and CD44. Immunity 2007, 26, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Huma, Z.E.; Sanchez, J.; Lim, H.D.; Bridgford, J.L.; Huang, C.; Parker, B.J.; Pazhamalil, J.G.; Porebski, B.T.; Pfleger, K.D.G.; Lane, J.R.; et al. Key determinants of selective binding and activation by the monocyte chemoattractant proteins at the chemokine receptor CCR2. Sci. Signal. 2017, 10, eaai8529. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, T.J.; Macdonald, S.J.F.; Peace, S.; Pickett, S.D.; Luscombe, C.N. Increasing small molecule drug developability in sub-optimal chemical space. Medchemcomm 2013, 4, 673. [Google Scholar] [CrossRef]
- Crijns, H.; Adyns, L.; Ganseman, E.; Cambier, S.; Vandekerckhove, E.; Pörtner, N.; Vanbrabant, L.; Struyf, S.; Gerlza, T.; Kungl, A.; et al. Affinity and Specificity for Binding to Glycosaminoglycans Can Be Tuned by Adapting Peptide Length and Sequence. Int. J. Mol. Sci. 2021, 23, 447. [Google Scholar] [CrossRef]
- Lagorce, D.; Douguet, D.; Miteva, M.A.; Villoutreix, B.O. Computational analysis of calculated physicochemical and ADMET properties of protein-protein interaction inhibitors. Sci. Rep. 2017, 7, 46277. [Google Scholar] [CrossRef] [PubMed]
- Bernimoulin, M.P.; Zeng, X.-L.; Abbal, C.; Giraud, S.; Martinez, M.; Michielin, O.; Schapira, M.; Spertini, O. Molecular Basis of Leukocyte Rolling on PSGL-1. J. Biol. Chem. 2003, 278, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldron, T.T.; Springer, T.A. Transmission of allostery through the lectin domain in selectin-mediated cell adhesion. Proc. Natl. Acad. Sci. USA 2009, 106, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorlacius, H.; Vollmar, B.; Seyfert, U.T.; Vestweber, D.; Menger, M.D. The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and l-selectin function in vivo. Eur. J. Clin. Investig. 2000, 30, 804–810. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Jarnagin, K.; Grunberger, D.; Mulkins, M.; Wong, B.; Hemmerich, S.; Paavola, C.; Bloom, A.; Bhakta, S.; Diehl, F.; Freedman, R.; et al. Identification of Surface Residues of the Monocyte Chemotactic Protein 1 That Affect Signaling through the Receptor CCR2. Biochemistry 1999, 38, 16167–16177. [Google Scholar] [CrossRef]
- Hemmerich, S.; Paavola, C.; Bloom, A.; Bhakta, S.; Freedman, R.; Grunberger, D.; Krstenansky, J.; Lee, S.; McCarley, D.; Mulkins, M.; et al. Identification of Residues in the Monocyte Chemotactic Protein-1 That Contact the MCP-1 Receptor, CCR2. Biochemistry 1999, 38, 13013–13025. [Google Scholar] [CrossRef]
- Joshi, N.; Tripathi, D.K.; Nagar, N.; Poluri, K.M. Hydroxyl Groups on Annular Ring-B Dictate the Affinities of Flavonol–CCL2 Chemokine Binding Interactions. ACS Omega 2021, 6, 10306–10317. [Google Scholar] [CrossRef]
- Yu, X.-H.; Zhang, J.; Zheng, X.-L.; Yang, Y.-H.; Tang, C.-K. Interferon-γ in foam cell formation and progression of atherosclerosis. Clin. Chim. Acta 2015, 441, 33–43. [Google Scholar] [CrossRef]
- Lee, J.-O.; Bankston, L.A.; Robert, C.; Liddington, M.A.A. Two conformations of the integrin A-domain (I-domain): A pathway for activation? Structure 1995, 3, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Shimaoka, M.; Xiao, T.; Liu, J.-H.; Yang, Y.; Dong, Y.; Jun, C.-D.; McCormack, A.; Zhang, R.; Joachimiak, A.; Takagi, J.; et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 2003, 112, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.P.; Fisher, K.L.; Presta, L.G.; Bodary, S.C. Mapping the Intercellular Adhesion Molecule-1 and -2 Binding Site on the Inserted Domain of Leukocyte Function-associated Antigen-1. J. Biol. Chem. 1998, 273, 28937–28944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, K.L.; Lu, J.; Riddle, L.; Kim, K.J.; Presta, L.G.; Bodary, S.C. Identification of the binding site in intercellular adhesion molecule 1 for its receptor, leukocyte function-associated antigen 1. Mol. Biol. Cell 1997, 8, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Rowe, A.; Berendt, A.R.; Marsh, K.; Newbold, C.I. Plasmodium falciparum: A Family of Sulfated Glycoconjugates Disrupts Erythrocyte Rosettes. Exp. Parasitol. 1994, 79, 506–516. [Google Scholar] [CrossRef]
- Skidmore, M.A.; Mustaffa, K.M.F.; Cooper, L.C.; Guimond, S.E.; Yates, E.A.; Craig, A.G. A semi-synthetic glycosaminoglycan analogue inhibits and reverses Plasmodium falciparum cytoadherence. PLoS ONE 2017, 12, e0186276. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Bella, J.; Kolatkar, P.R.; Marlor, C.W.; Greve, J.M.; Rossmann, M.G. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4140–4145. [Google Scholar] [CrossRef] [Green Version]
- Lubkowski, J.; Bujacz, G.; Boqué, L.; Peter, J.D.; Tracy, M.H.; Alexander, W. The Structure of MC P-1 in Two Crystal Forms Provides a Rare Example of Variable Quaternary Interactions. Nat. Struct. Biol. 1997, 4, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [Green Version]
- Yurdakok Dikmen, B.; Alpay, M.; Kismali, G.; Filazi, A.; Kuzukiran, O.; Sireli, U.T. In Vitro Effects of Phthalate Mixtures on Colorectal Adenocarcinoma Cell Lines. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Davies, T.S.; Garaiova, I.; Plummer, S.F.; Michael, D.R.; Ramji, D.P. A Unique Combination of Nutritionally Active Ingredients Can Prevent Several Key Processes Associated with Atherosclerosis In Vitro. PLoS ONE 2016, 11, e0151057. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Physicochemical Properties | Fucoidan | Alginate |
|---|---|---|
| MLogP | −1.49 | −2.89 |
| Molecular weight | 256.27 | 193.13 |
| Number of H-bond acceptors | 7 | 7 |
| Number of H-bond donors | 2 | 4 |
| Number Rotatable bonds | 3 | 1 |
| Protein | PDB ID | Ligand | Binding Energy (Kcal/mol) | Inhibition Constant (Ki) | Interacting Residues |
|---|---|---|---|---|---|
| L-selectin | 5VC1 | Fucoidan | −5.82 | 54.41 µM | Lys84(1.9Å), Glu88(1.9Å), Tyr94(2.6Å), Asn105 (2.0Å), Lys111(2.0Å), |
| Alginate | −4.3 | 704.72 µM | Lys55(2.2 Å), Trp60(2.6Å), Glu88(1.9 Å), | ||
| E-selectin | 1G1T | Fucoidan | −5.69 | 67.62 µM | Lys55(2.0Å), Asn58(2.1Å), Asn83(2.7Å), Arg84(2.6Å), Asp106(2.1Å) |
| Alginate | −4.09 | 997.16 µM | Asn58(1.9 Å), Trp60(2.0 Å), Lys74(1.9Å), Trp76(1.8 Å) | ||
| MCP-1 | 1DOK | Fucoidan | −5.67 | 69.96 µM | Cys11(1.8 Å), Tyr13(2.1 Å), Asn14(2.0Å), Cys52(1.9 Å) |
| Alginate | −3.84 | 1.52 mM | Asn14(2.0 Å), Glu50(2.1Å), Cys52(1.7Å) | ||
| ICAM-1 | 1IAM | Fucoidan | −5.66 | 70.39 µM | Leu33(1.7Å), Lys39(2.4Å), Glu41(1.8Å), Lys50(1.8Å), Tyr52(1.9Å), Tyr66(2.1Å) |
| Alginate | −4.98 | 224.33 µM | Leu33(1.9Å), Lys39(1.9Å), Glu41(2.3 Å), Lys50(2.5Å), Tyr52(2.1Å), Tyr66(2.2Å) |
| Gene | Primer Sequence |
|---|---|
| MCP-1 | Forward: CGCTCAGCCAGATGCAATCAATG Reverse: CGCTCAGCCAGATGCAATCAATG |
| ICAM-1 | Forward: GACCAGAGGTTGAACCCCAC Reverse: GCGCCGGAAAGCTGTAGAT |
| GAPDH | Forward: CTTTTGCGTCGCCAGCCGAG Reverse: GCCCAATACGACCAAATCCGTTGACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwait, E.; Al-Saedi, D.A.; Mirza, Z. Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and In Vitro Studies in THP-1 Cells. Molecules 2022, 27, 3197. https://doi.org/10.3390/molecules27103197
Huwait E, Al-Saedi DA, Mirza Z. Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and In Vitro Studies in THP-1 Cells. Molecules. 2022; 27(10):3197. https://doi.org/10.3390/molecules27103197
Chicago/Turabian StyleHuwait, Etimad, Dalal A. Al-Saedi, and Zeenat Mirza. 2022. "Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and In Vitro Studies in THP-1 Cells" Molecules 27, no. 10: 3197. https://doi.org/10.3390/molecules27103197
APA StyleHuwait, E., Al-Saedi, D. A., & Mirza, Z. (2022). Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and In Vitro Studies in THP-1 Cells. Molecules, 27(10), 3197. https://doi.org/10.3390/molecules27103197